Pick which of the following pairs will have the higher boiling point. Then, determine why. Pick the best choice. (Please see attached image) Which has the highest boiling point? Molecule on the top Molecule on the bottom Both will have the same boiling point Why? it has a bigger molecular weight they both have the same intermolecular forces, but one has a bigger molecular weight its strongest intermolecular force is dipole-dipole forces its strongest intermolecular force is hydrogen bonding
Pick which of the following pairs will have the higher boiling point. Then, determine why. Pick the best choice. (Please see attached image) Which has the highest boiling point? Molecule on the top Molecule on the bottom Both will have the same boiling point Why? it has a bigger molecular weight they both have the same intermolecular forces, but one has a bigger molecular weight its strongest intermolecular force is dipole-dipole forces its strongest intermolecular force is hydrogen bonding
Chapter22: Organic And Biological Molecules
Section: Chapter Questions
Problem 57E
Related questions
Question
Pick which of the following pairs will have the higher boiling point. Then, determine why. Pick the best choice. (Please see attached image)
Which has the highest boiling point?
- Molecule on the top
- Molecule on the bottom
- Both will have the same boiling point
Why?
- it has a bigger molecular weight
- they both have the same intermolecular forces, but one has a bigger molecular weight
- its strongest intermolecular force is dipole-dipole forces
- its strongest intermolecular force is hydrogen bonding
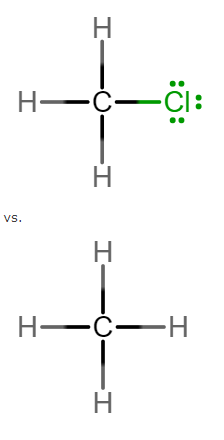
Transcribed Image Text:H.
vs.
Н—с—н
H
エー
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
