ion Part'2 What is the molar mass of oACetone 2 Pound yourC ansuef w four dignificant figures- to part 3 answer ro four signiticant figures. oनिकववेम् ४|0जठ न-ट उ० जऽ0 w ত Two major factors influence the boiling points and vapor pressures of molecules: molar mass and strength of intermolecular forces. Consider the electrostatic potential maps for the three molecules shown. Methyl propane Acetone 2-Propanol
ion Part'2 What is the molar mass of oACetone 2 Pound yourC ansuef w four dignificant figures- to part 3 answer ro four signiticant figures. oनिकववेम् ४|0जठ न-ट उ० जऽ0 w ত Two major factors influence the boiling points and vapor pressures of molecules: molar mass and strength of intermolecular forces. Consider the electrostatic potential maps for the three molecules shown. Methyl propane Acetone 2-Propanol
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter3: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 22A
Related questions
Question

Transcribed Image Text:ion
Part'2
What is the molar mass of oACetone 2 Pound yourC ansuef
w four dignificant figures-
to
part 3
answer ro four signiticant figures.
oनिकववेम् ४|0जठ न-ट उ० जऽ0 w ত
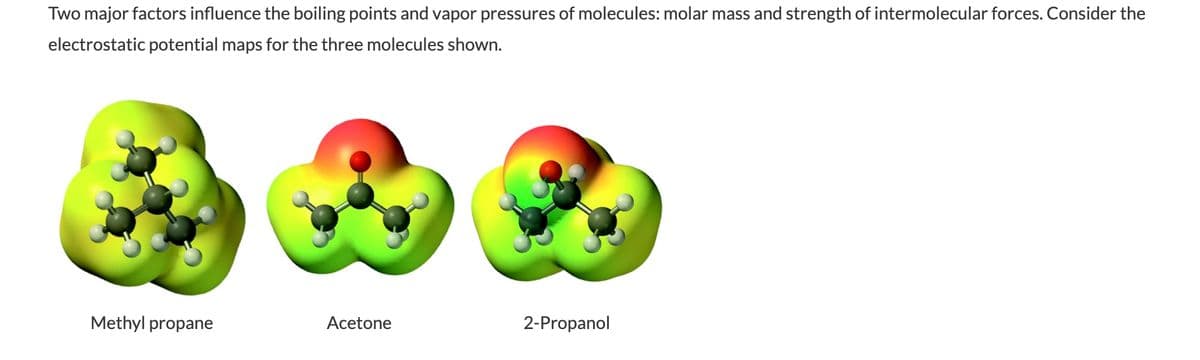
Transcribed Image Text:Two major factors influence the boiling points and vapor pressures of molecules: molar mass and strength of intermolecular forces. Consider the
electrostatic potential maps for the three molecules shown.
Methyl propane
Acetone
2-Propanol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning