Pill 1 Pill 2 Mass percent of FESO4 7 H,O (%) Average mass percent of FESO, 7 H2O (%) 6. unrounded Average mass percent of FeSO4 7 H,O (%) rounded
Pill 1 Pill 2 Mass percent of FESO4 7 H,O (%) Average mass percent of FESO, 7 H2O (%) 6. unrounded Average mass percent of FeSO4 7 H,O (%) rounded
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.30QAP
Related questions
Question
100%
Can someone please help compete the last table
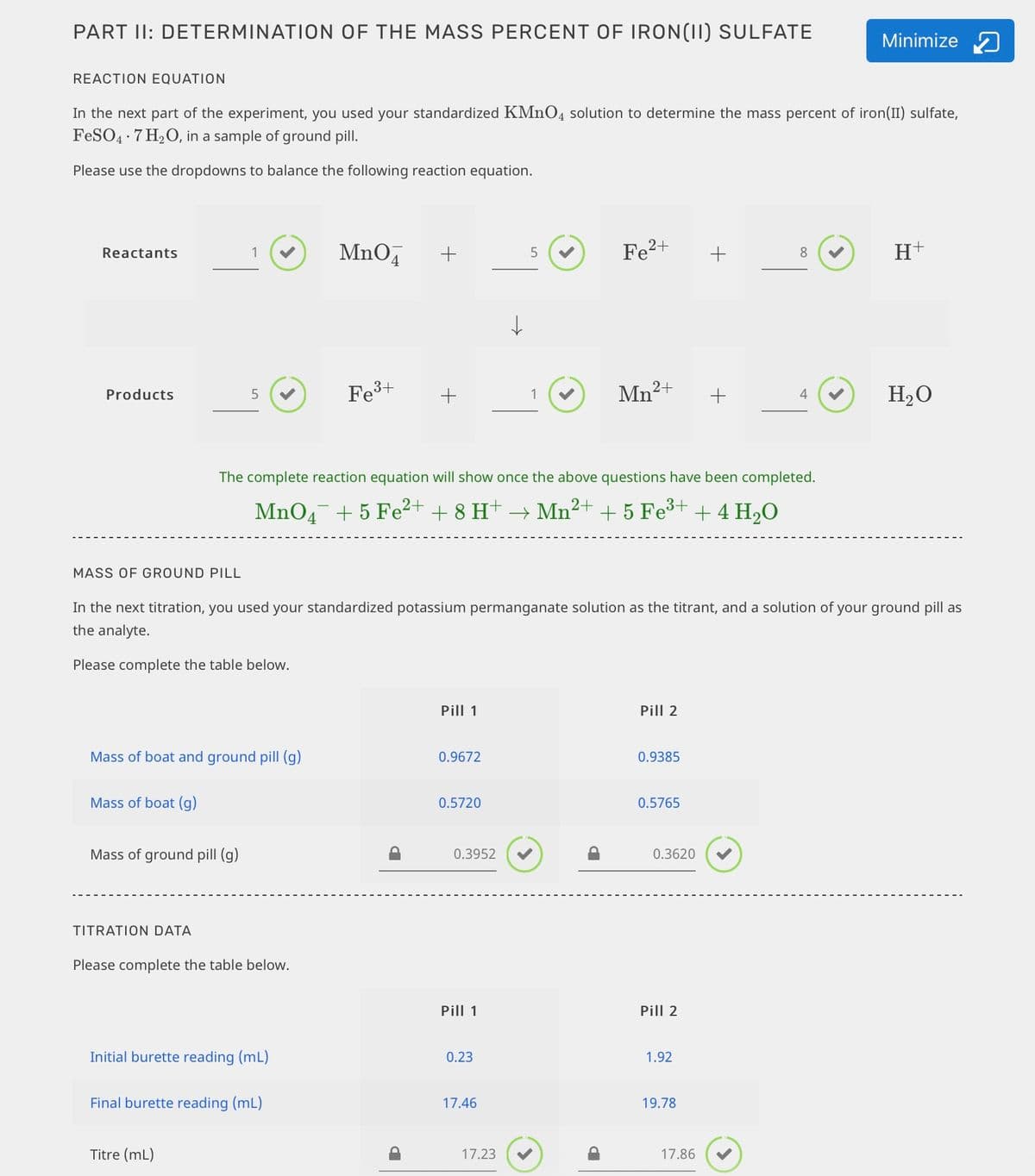
Transcribed Image Text:PART II: DETERMINATION OF THE MASS PERCENT OF IRON(II) SULFATE
Minimize a
REACTION EQUATION
In the next part of the experiment, you used your standardized KMNO4 solution to determine the mass percent of iron(II) sulfate,
FeSO4 · 7 H2O, in a sample of ground pill.
Please use the dropdowns to balance the following reaction equation.
MnO,
Fe2+
H+
Reactants
1
5
8
Fe3+
Mn²
2+
+
H2O
Products
4
The complete reaction equation will show once the above questions have been completed.
2+
MnO4¯ + 5 Fe2+
+ 8 H+ → Mn²
+ 5 Fe3+
+ 4 H2O
MASS OF GROUND PILL
In the next titration, you used your standardized potassium permanganate solution as the titrant, and a solution of your ground pill as
the analyte.
Please complete the table below.
Pill 1
Pill 2
Mass of boat and ground pill (g)
0.9672
0.9385
Mass of boat (g)
0.5720
0.5765
Mass of ground pill (g)
0.3952
0.3620
TITRATION DATA
Please complete the table below.
Pill 1
Pill 2
Initial burette reading (mL)
0.23
1.92
Final burette reading (mL)
17.46
19.78
Titre (mL)
17.23
17.86
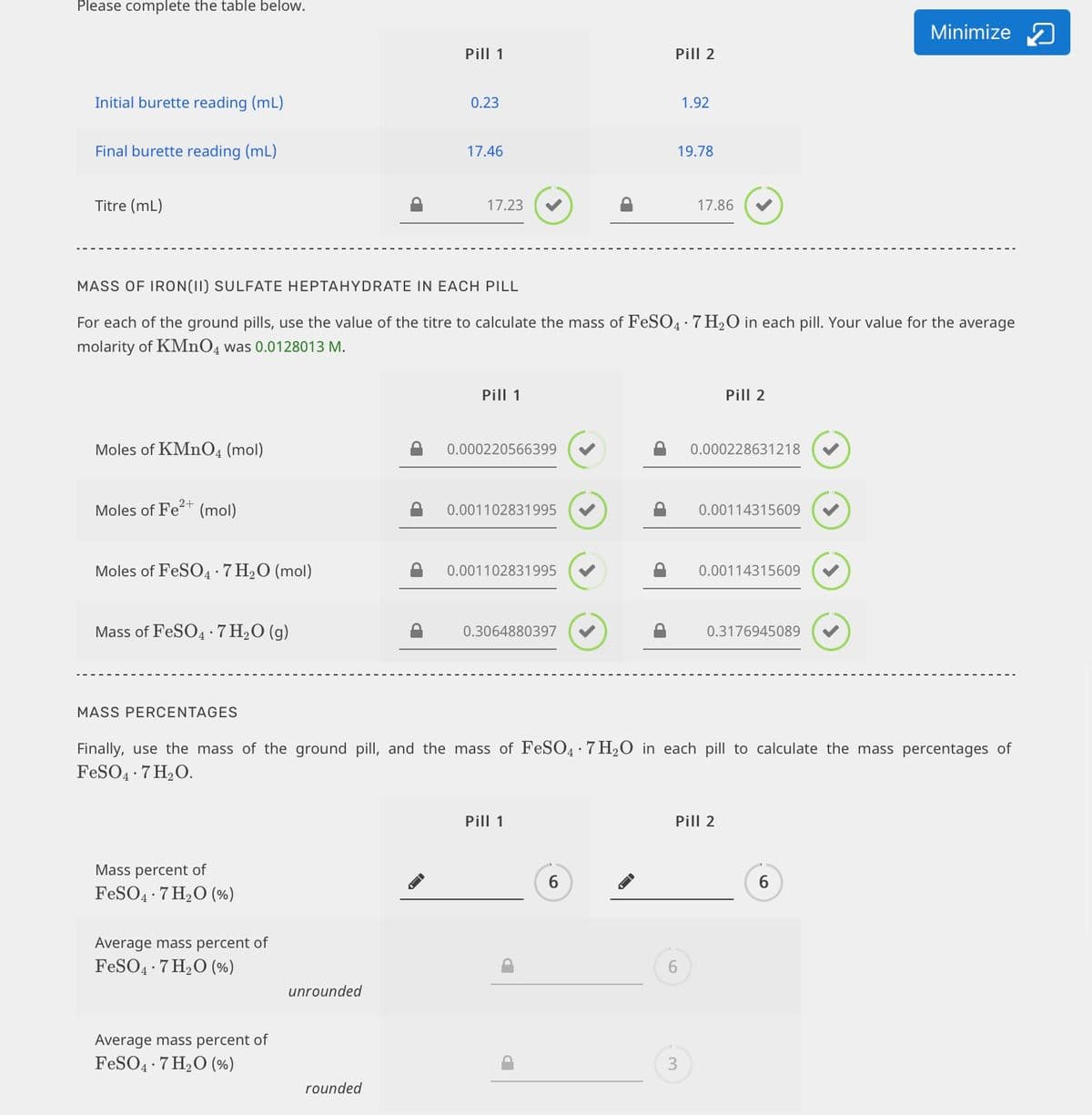
Transcribed Image Text:Please complete the table below.
Minimize D
Pill 1
Pill 2
Initial burette reading (mL)
0.23
1.92
Final burette reading (mL)
17.46
19.78
Titre (mL)
17.23
17.86
MASS OF IRON(II) SULFATE HEPTAHYDRATE IN EACH PILL
For each of the ground pills, use the value of the titre to calculate the mass of FeSO4:7 H2O in each pill. Your value for the average
molarity of KMNO4 was 0.0128013 M.
Pill 1
Pill 2
Moles of KMNO4 (mol)
0.000220566399
0.000228631218
Moles of Fe+ (mol)
0.001102831995
0.00114315609
Moles of FeSO4·7 H2O (mol)
0.001102831995
0.00114315609
Mass of FeSO4 · 7 H2O (g)
0.3064880397
0.3176945089
MASS PERCENTAGES
Finally, use the mass of the ground pill, and the mass of FeSO4 · 7 H2O in each pill to calculate the mass percentages of
FeSO4 7 H2O.
Pill 1
Pill 2
Mass percent of
6.
FeSO4 · 7 H2O (%)
Average mass percent of
FeSO4 · 7 H2O (%)
6.
unrounded
Average mass percent of
FeSO4 · 7 H,O (%)
3.
rounded
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning