Please complete the following table. For each of your trials, enter the mass of crushed antacid tablet (g) dissolved in HCl, the volume of HCl (ml) used to dissolve the sample, the corresponding volume of NaOH (mL) required to reach the endpoint, and the calculated mmoles of HCl needed to neutralize 1 mg of sample. Use the average molarity of your NaOH solution and the average molarity of your HCl solution in your calculations.
Please complete the following table. For each of your trials, enter the mass of crushed antacid tablet (g) dissolved in HCl, the volume of HCl (ml) used to dissolve the sample, the corresponding volume of NaOH (mL) required to reach the endpoint, and the calculated mmoles of HCl needed to neutralize 1 mg of sample. Use the average molarity of your NaOH solution and the average molarity of your HCl solution in your calculations.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter16: Acids And Bases
Section: Chapter Questions
Problem 2STP
Related questions
Question
Average HCl
0.421203
Average M NaOH
0.0900235
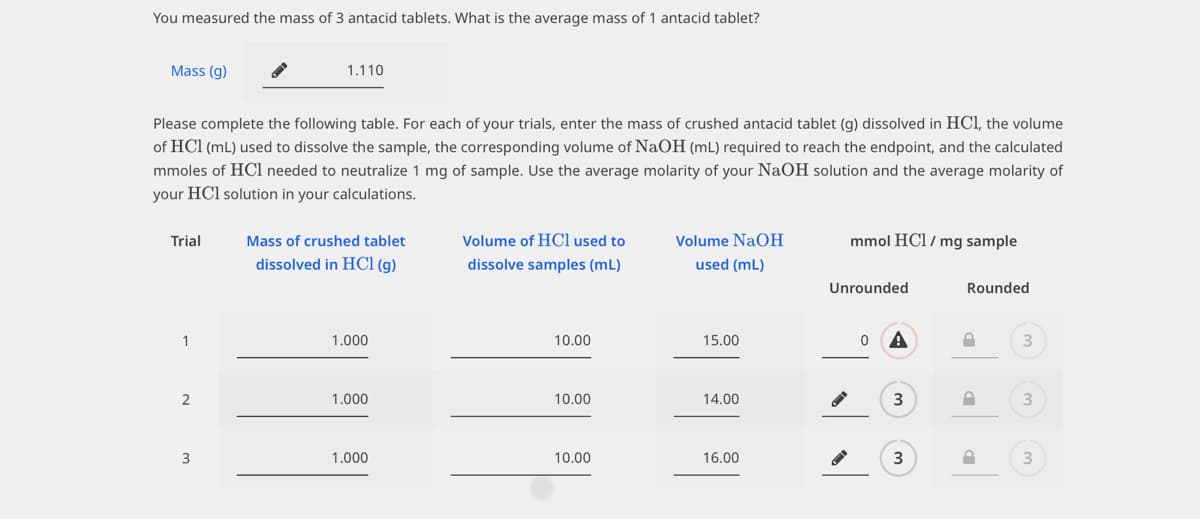
Transcribed Image Text:You measured the mass of 3 antacid tablets. What is the average mass of 1 antacid tablet?
Mass (g)
Please complete the following table. For each of your trials, enter the mass of crushed antacid tablet (g) dissolved in HCl, the volume
of HCl (ml) used to dissolve the sample, the corresponding volume of NaOH (mL) required to reach the endpoint, and the calculated
mmoles of HCl needed to neutralize 1 mg of sample. Use the average molarity of your NaOH solution and the average molarity of
your HCl solution in your calculations.
Trial
1
2
1.110
3
Mass of crushed tablet
dissolved in HCl (g)
1.000
1.000
1.000
Volume of HCl used to
dissolve samples (ml)
10.00
10.00
10.00
Volume NaOH
used (ml)
15.00
14.00
16.00
mmol HCl / mg sample
Unrounded
0 A
3
3
Rounded
3
3
3
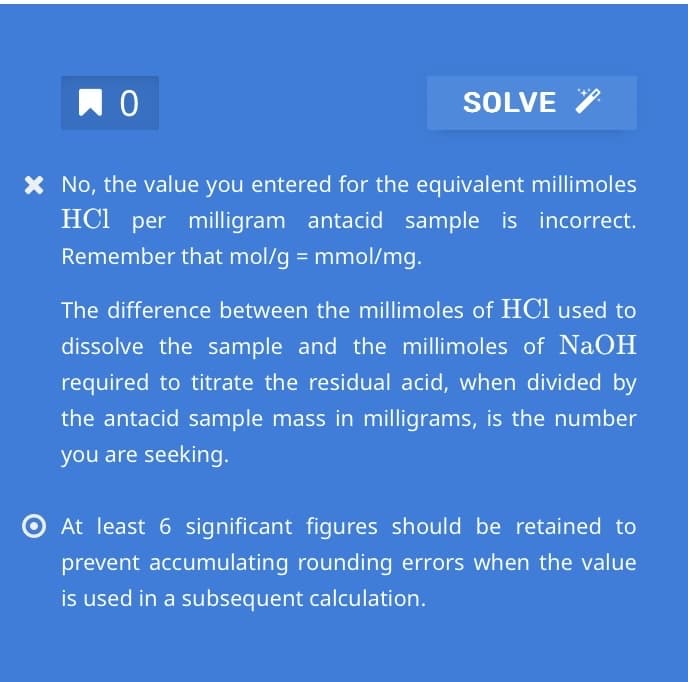
Transcribed Image Text:по
SOLVE
* No, the value you entered for the equivalent millimoles
HCl per milligram antacid sample is incorrect.
Remember that mol/g = mmol/mg.
The difference between the millimoles of HCl used to
dissolve the sample and the millimoles of NaOH
required to titrate the residual acid, when divided by
the antacid sample mass in milligrams, is the number
you are seeking.
At least 6 significant figures should be retained to
prevent accumulating rounding errors when the value
is used in a subsequent calculation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
