Please help answering the all parts of the following question...thank a.) Given that the condensed molecular formula for the following compound, C5H12O, predict the bonds you would expect this molecule to have based on the given IR spectrum. Draw arrows to the peaks with the bond(s) they represent. b.) Based on your findings from the IR spectrum, propose 3 different constitutional isomers that are consistent with your findings.
Please help answering the all parts of the following question...thank a.) Given that the condensed molecular formula for the following compound, C5H12O, predict the bonds you would expect this molecule to have based on the given IR spectrum. Draw arrows to the peaks with the bond(s) they represent. b.) Based on your findings from the IR spectrum, propose 3 different constitutional isomers that are consistent with your findings.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.21PAE: 1.21 When a scientist looks at an experiment and then predicts the results of other related...
Related questions
Question
Please help answering the all parts of the following question...thank
a.) Given that the condensed molecular formula for the following compound, C5H12O, predict the bonds you would expect this molecule to have based on the given IR spectrum. Draw arrows to the peaks with the bond(s) they represent.
b.) Based on your findings from the IR spectrum, propose 3 different constitutional isomers that are consistent with your findings.
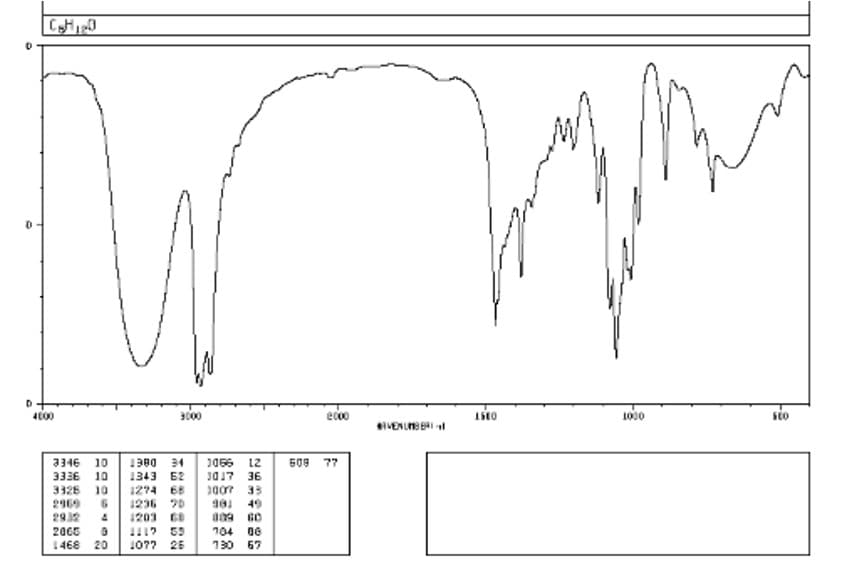
Transcribed Image Text:4000
300
1000
Sto
3346
10
1980
J066
12
508 77
3336
10
1343 62
36
3126
2969
10
1274 68
33
49
1296 70
1203 CB
2932
2000
1468 20
GO
55
107? 25
104
130 67
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning