Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 25P
Related questions
Question
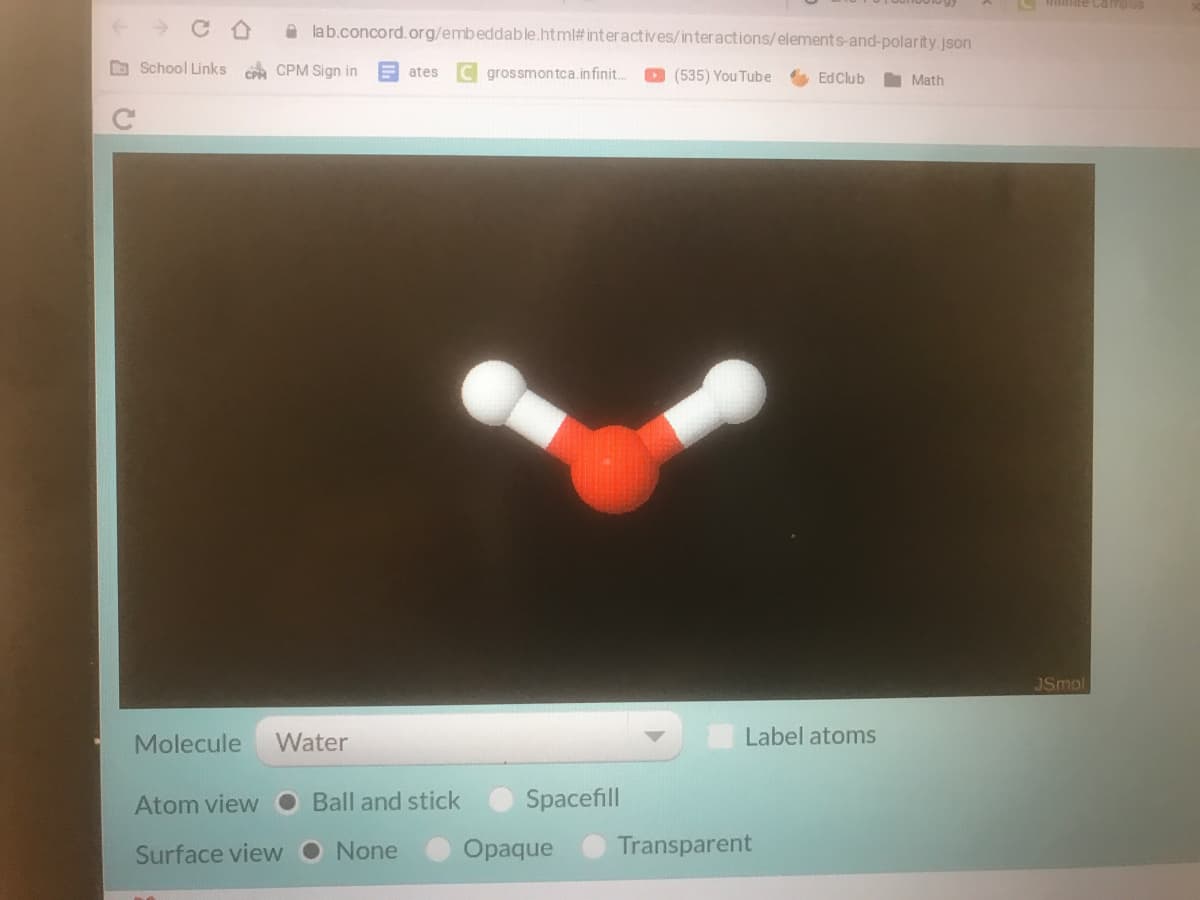
Please i need to answer all the questions. And for question six her is a picture of the model
Transcribed Image Text:mnte Campus
a lab.concord.org/embeddable.html#interactives/interactions/elements-and-polarity.json
D School Links
c CPM Sign in
E ates
C grossmon tca.in finit.
(535) You Tube
EdClub
Math
JSmol
Molecule
Water
Label atoms
Atom view
Ball and stick
Spacefill
Surface view O None
Opaque
Transparent
Transcribed Image Text:pen Sans
BIUA
12
== 1三 ニ三。
E EEX
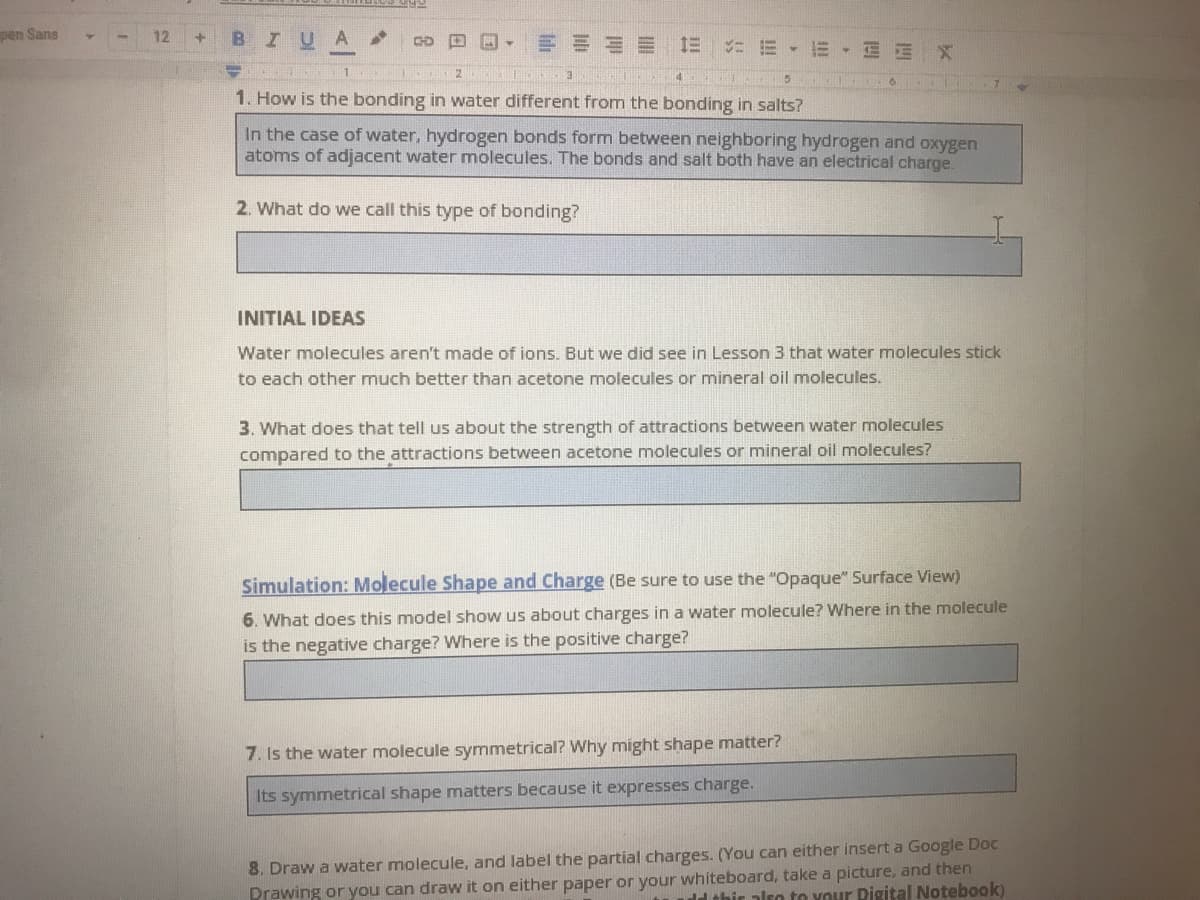
1. How is the bonding in water different from the bonding in salts?
In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen
atoms of adjacent water molecules. The bonds and salt both have an electrical charge
2. What do we call this type of bonding?
INITIAL IDEAS
Water molecules aren't made of ions. But we did see in Lesson 3 that water molecules stick
to each other much better than acetone molecules or mineral oil molecules.
3. What does that tell us about the strength of attractions between water molecules
compared to the attractions between acetone molecules or mineral oil molecules?
Simulation: Molecule Shape and Charge (Be sure to use the "Opaque" Surface View)
6. What does this model show us about charges in a water molecule? Where in the molecule
is the negative charge? Where is the positive charge?
7. Is the water molecule symmetrical? Why might shape matter?
Its symmetrical shape matters because it expresses charge.
8. Draw a water molecule, and label the partial charges. (You can either insert a Google Doc
Prawing or you can draw it on either paper or your whiteboard, take a picture, and then
ld thir also to your Pigital Notebook)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning