Potassium (K) reacts with oxygen gas (O,) to form potassium oxide (K,O). Which equation for this reaction is correctly balanced? K+ O2 K,O 2K + O2 K20 4K + O2 2K,0 4K+202 2K,0 B 26 27 28 29 30 31 33 34 35 Save/Exit
Potassium (K) reacts with oxygen gas (O,) to form potassium oxide (K,O). Which equation for this reaction is correctly balanced? K+ O2 K,O 2K + O2 K20 4K + O2 2K,0 4K+202 2K,0 B 26 27 28 29 30 31 33 34 35 Save/Exit
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
Transcribed Image Text:OnCourse Connect
Assessment: Chemistry x
4 Copy of spread of jslar x
E The Spread of Islam - G x
M Inbox (904) - aarojame: x
A Classes
oncourseconnect.com/assessment/1651879/5287e2a3-0d0b-e2c0-c15c-3e68a37149b4
O TPSS Bookmarks
CHEMISTRY BENCHMARK TEST 01
CHEMISTRY I 12-7 (AARON JAMES, ID: 12390724)
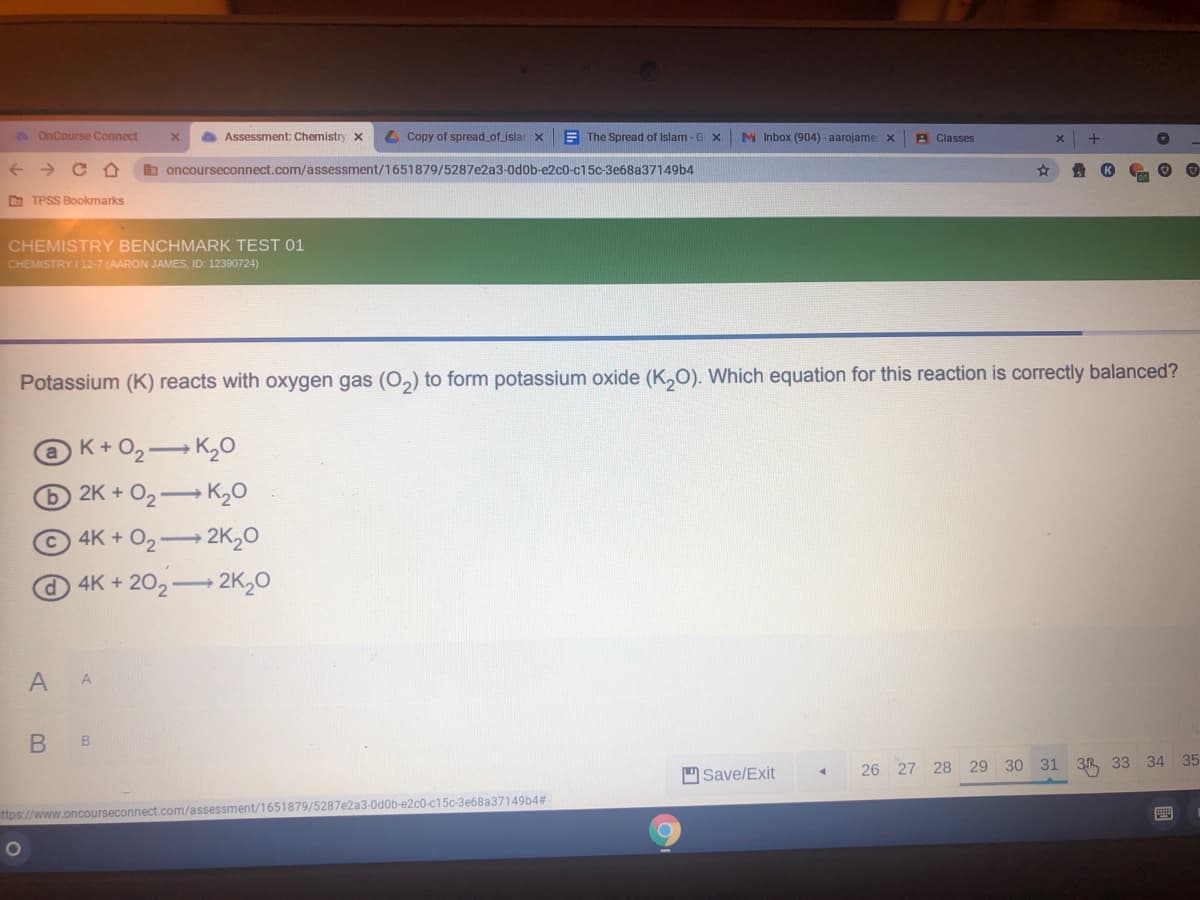
Potassium (K) reacts with oxygen gas (O,) to form potassium oxide (K,O). Which equation for this reaction is correctly balanced?
K+O2 K,0
2K + O2 K20
4K+ O2 2K20
4K+202 2K,0
35
Save/Exit
26 27 28 29 30 31 3R 33 34
ttps://www.oncourseconnect.com/assessment/1651879/5287e2a3-0d0b-e2c0-c15c-3e68a37149b4#
B.
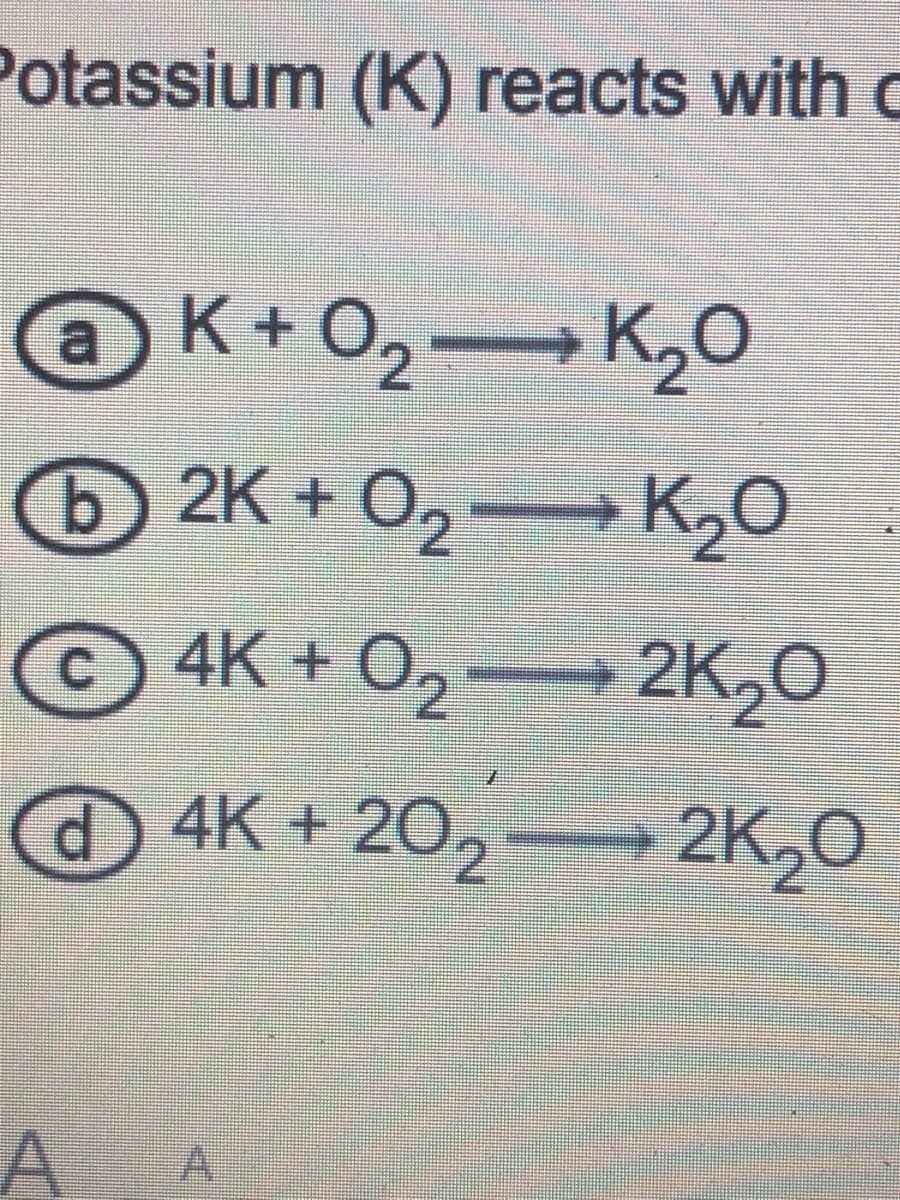
Transcribed Image Text:Potassium (K) reacts with c
K+O2 K20
2K + O2→ K2O
© 4K + O2
+ 2K20
@4K + 20,
2K20
A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

