Potentially Useful Reference Information Kw = 0.114 x 10-14 at 0°C Kw = 0.465 x 10-14 at 15°C Kw = 0.681 x 10´14 at 20°C Kw = 1.00 x 10 14 at 25°C Kw = 2.42 x 10-14 at 37°C Kw = 9.61 x 10-14 at 60°C Acid Ka HCI 1.3 x 106 H2SO4 1 x 103 HNO3 2.4 x 101 HSO4 1.2 x 10-2 H3PO4 7.5 x 10-3 A student measures the temperature of an NaOH solution to be 15 degrees C. The student then measures the pH of this NaOH solution to be pH = 12.39. H3CCOOH 1.8 x 10-5 H2CO3 4.3 x 10-7 H2PO4 6.2 x 10-8 Determine the molarity of this sodium hydroxide solution at 15°C. Error analysis is not necessary, but report your answer with 2 HCO3 4.8 x 10-11 significant figures. HPO,2- 4.8 x 1013
Potentially Useful Reference Information Kw = 0.114 x 10-14 at 0°C Kw = 0.465 x 10-14 at 15°C Kw = 0.681 x 10´14 at 20°C Kw = 1.00 x 10 14 at 25°C Kw = 2.42 x 10-14 at 37°C Kw = 9.61 x 10-14 at 60°C Acid Ka HCI 1.3 x 106 H2SO4 1 x 103 HNO3 2.4 x 101 HSO4 1.2 x 10-2 H3PO4 7.5 x 10-3 A student measures the temperature of an NaOH solution to be 15 degrees C. The student then measures the pH of this NaOH solution to be pH = 12.39. H3CCOOH 1.8 x 10-5 H2CO3 4.3 x 10-7 H2PO4 6.2 x 10-8 Determine the molarity of this sodium hydroxide solution at 15°C. Error analysis is not necessary, but report your answer with 2 HCO3 4.8 x 10-11 significant figures. HPO,2- 4.8 x 1013
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
SectionU5.9: Counting Calories: Calorimetry Calculations
Problem 4E
Related questions
Question
100%
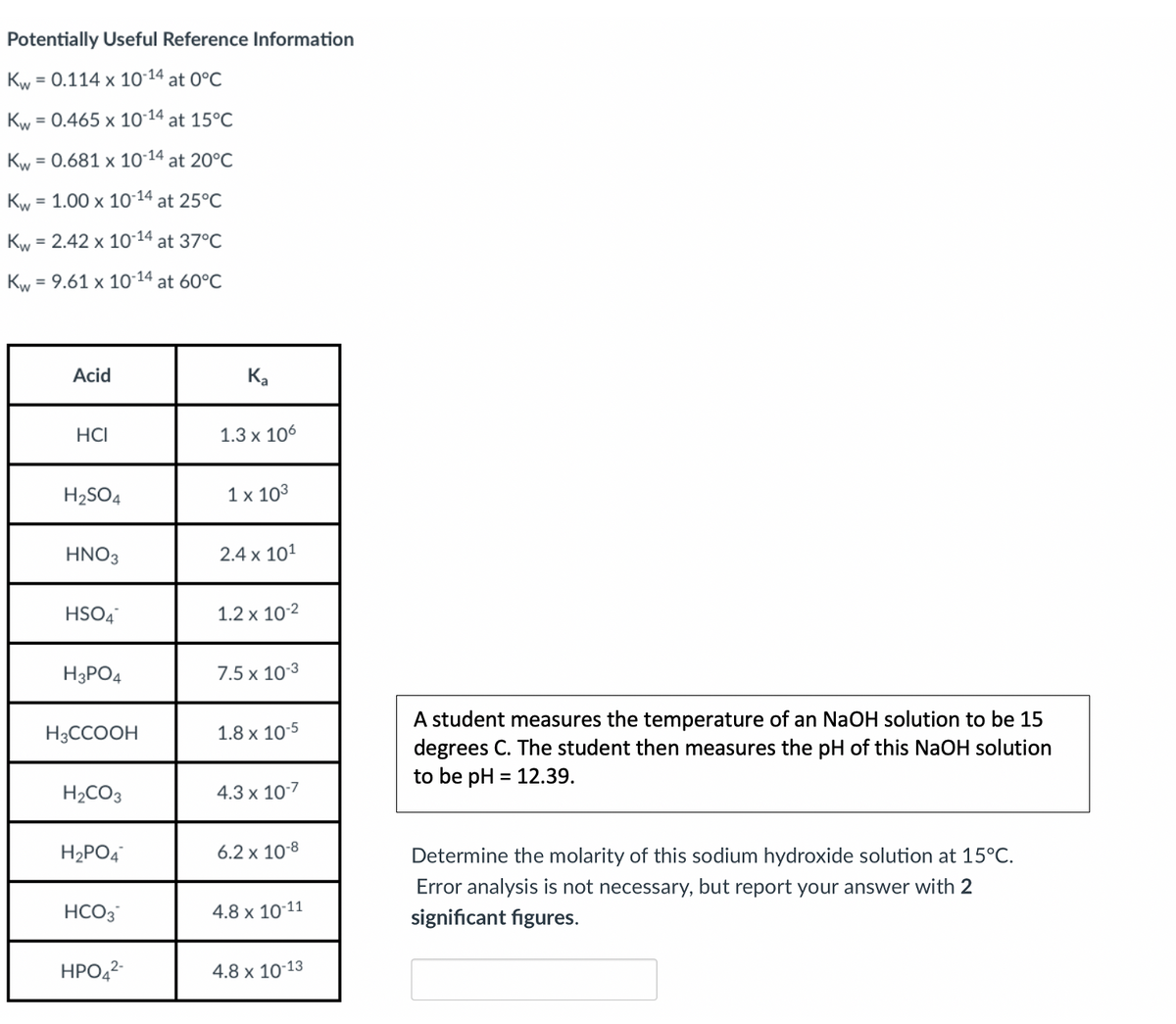
Transcribed Image Text:Potentially Useful Reference Information
Kw
= 0.114 x 10-14 at 0°C
Kw
= 0.465 x 1014 at 15°C
Kw
= 0.681 x 10-14
at 20°C
Kw
= 1.00 x 10-14 at 25°C
Kw
= 2.42 x 10-14 at 37°C
Kw
= 9.61 x 1014 at 60°C
Acid
Ka
HCI
1.3 x 106
H2SO4
1 x 103
HNO3
2.4 x 101
HSO4
1.2 x 10-2
H3PO4
7.5 x 103
A student measures the temperature of an NaOH solution to be 15
degrees C. The student then measures the pH of this NaOH solution
to be pH = 12.39.
H3CCOOH
1.8 x 10-5
%3D
H2CO3
4.3 x 10:7
H2PO4
6.2 x 10-8
Determine the molarity of this sodium hydroxide solution at 15°C.
Error analysis is not necessary, but report your answer with 2
HCO3
4.8 x 10-11
significant figures.
HPO,2
4.8 x 10-13
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER