Part I: Calibration of the Plastic Pipette Number of Drops Mass of 10 Drops (9) Volume of 10 Total Mass (g) Drops (mL) 23.3124 10 23.8216 20 24.2999 30 24.8245 24.7 1. Temperature of deionized water (°C) 2. Density of water at this temperature from (g/mL) Density calculator https://antoine.frostburg.edu/chem/senese/javascript/water-density.html 3. Average volume of 10 drops (mL) show calculation:
Part I: Calibration of the Plastic Pipette Number of Drops Mass of 10 Drops (9) Volume of 10 Total Mass (g) Drops (mL) 23.3124 10 23.8216 20 24.2999 30 24.8245 24.7 1. Temperature of deionized water (°C) 2. Density of water at this temperature from (g/mL) Density calculator https://antoine.frostburg.edu/chem/senese/javascript/water-density.html 3. Average volume of 10 drops (mL) show calculation:
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
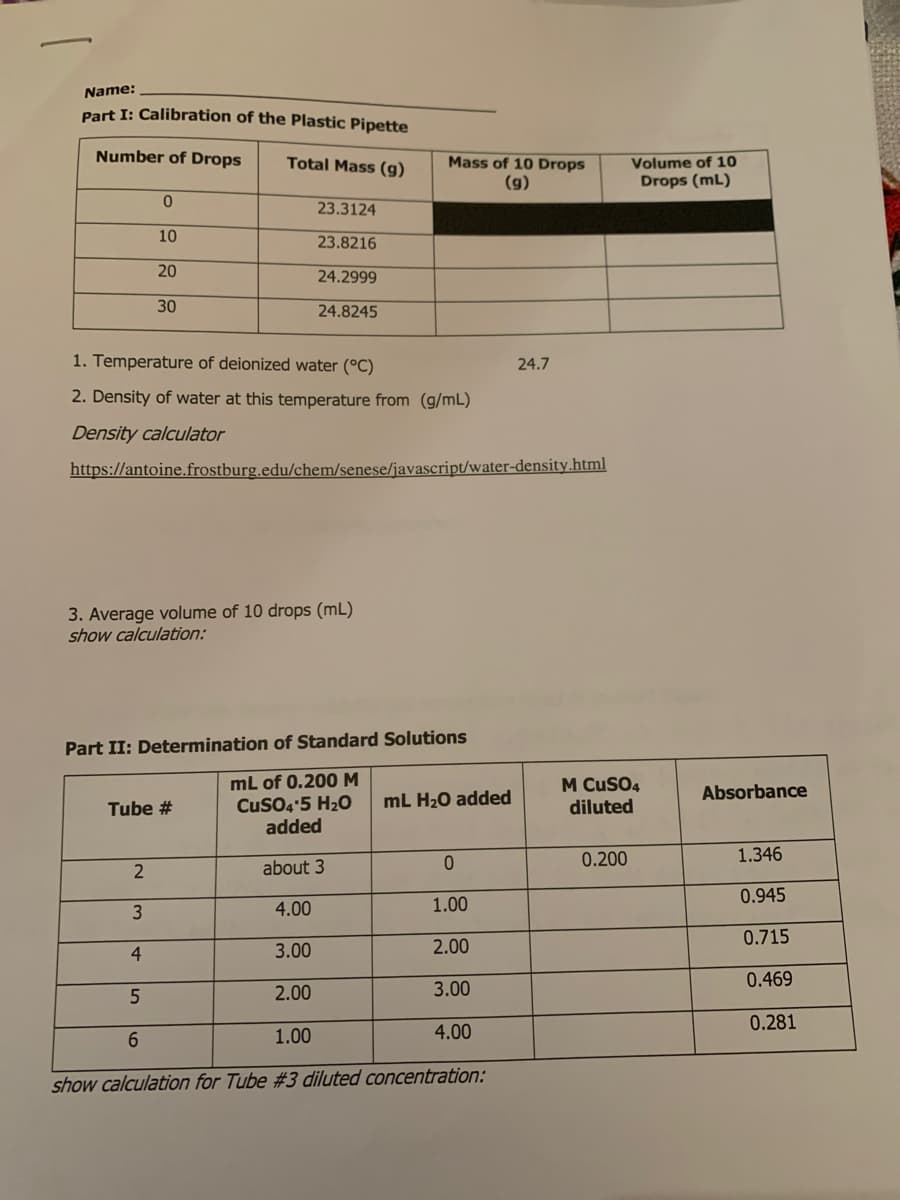
Transcribed Image Text:Name:
Part I: Calibration of the Plastic Pipette
Number of Drops
Total Mass (g)
Mass of 10 Drops
(g)
Volume of 10
Drops (mL)
23.3124
10
23.8216
20
24.2999
30
24.8245
1. Temperature of deionized water (°C)
24.7
2. Density of water at this temperature from (g/mL)
Density calculator
https://antoine.frostburg.edu/chem/senese/javascript/water-density.html
3. Average volume of 10 drops (mL)
show calculation:
Part II: Determination of Standard Solutions
mL of 0.200M
CuSO4'5 H20
added
M CuSO4
diluted
Absorbance
Tube #
mL H20 added
0.200
1.346
about 3
0.945
3
4.00
1.00
0.715
4
3.00
2.00
0.469
2.00
3.00
0.281
6.
1.00
4.00
show calculation for Tube #3 diluted concentration:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

