AutoSave OFF Document1 Home Insert Draw Design Layout References Mailings Review View Acrobat Tell me Share Comments Calibri (Bo... A A Аa v E v E - E v 12 >T A Styles Styles Pane Dictate Sensitivity Create and Share Adobe PDF Paste A v Request Signatures U v ab x, x -CH, -CH(CH,), -CH,CI -CH-CH, -CH=CHAr -0.18 -0.11 -0.21 -0,14 0.02 -0.08 --0.20 -0.01 Typical proton-proton coupling constants -0.04 TABLE 22.6 -0.12 -0.11 0.04 -0.04 0.14 -0.02 Arrangment of protons J(Hz) Arrangement protons J(Hz) Arrangement of protons J(Hz) -CH=CHCO,H -CH=CH(C=O)Ar 0.19 0.04 0.05 0.28 0.06 0.05 (Continued) 10 to 16 O to 3 Free rotation H. -H 8 to 13 11 to 14 12 to 18 H r Magnetic Resonance Spectroscopy 371 Anti H. Group ortho meta para -Ar 0.23 0.07 -0.02 2 to 4 8 to 13 6 to 12 -(C=O)H -(C-O)R 0.53 0.18 0.28 0.60 0.10 0.20 Gauche H. -(C=O)Ar 0.45 0.12 0.23 -(C-O)CH=CHA. -(C=O)OCH, -(C=0)OCH,CH, (C=O)OH -(C=0)CI -(C=O)NH, -CEN -F 0.67 0.68 0.14 0.21 0.08 0.19 0.69 0.06 0.17 0.77 0.11 0.25 6 to 9 2 to 6 4 to 10 0.76 0.16 0.33 0.46 0.09 0.17 0.29 0.12 0.25 -0.32 -0.05 -0.25 -0.13 -0.08 -CI -0.02 -0.07 -Br 0.13 -0.13 1 to 3 2 to 5 0.5 to 2 -0.43 -0.41 -OH -0.53 -0.14 -OR -0.45 -0.07 -OAr -0.36 -0.04 -0.28 -O(C=O)R -O(C=O)Ar -0.27 0.02 -0.13 -0.14 -0.71 -0.68 0.07 -0.09 -NH, -N(CH,), -NH(C=O)R -NO, -0.22 -0.62 O to 1 -0.15 -0.73 H. H. 0.14 -0.07 -0.27 0.87 0.20 0.35 a. Base value is the measured chemical shift of benzene in CDCI, (1% solution). Additive Parameters for Predicting NMR Chemical Shifts of Vinyl Protons in CDCI,a TABLE 22. 5 cis H Additive parameters for predicting NMR chemical shifts of alkyl protons in CDCI, TABLE 22.3 Base values trans gem Methyl Methylene Methine 0.9 ppm 1.2 ppm 1.5 ppm Base value 5.28 ppm Group (Y) Alpha (a) substituent Beta (B) substituent Gamma (y) substituent Group gem cis trans -R 0.45 -0.22 -0.28 H- -Y H- -Y H -0.01 -CH=CH, –CH,OH -CH,X (X=F, CI, Br) {C=O)OH -(C=O)OR 1.26 0.08 -R 0.0 0.0 0.64 -0.02 -0.04 0.0 -0.01 -C=C -C=C-Ar -C=C(C=O)OR -C=C-R 0.8 0.2 0.1 0.70 -0.11 0.9 0.1 0.0 0.71 0.55 1.0 0.3 0.1 0.97 1.41 0.9 0.3 0.1 0.80 1.18 -C=C-Ar 1.2 0.4 0.2 -(C=O)H -(C=0)R -Ar 1.4 0.4 0.1 1.02 0.95 1.17 -(C=O)OH -(C=O)OR -(C=O)H -(C=O)R -(C=O)An -(C=O)NH, -(C=O)CI -CEN 1.1 0.3 0.3 0.1 1.10 1.12 1.13 0.87 1.1 0.1 -(C=0)Ar -Ar 1.1 0.4 0.1 1.82 0.63 1.2 0.3 0.0 1.38 0.36 -0.07 1.7 0.3 0.1 0.45 0.18 -Br 1.07 0.55 1.0 0.3 0.1 -CI -OR 1.8 0.4 0.1 1.08 0.13 1.1 0.4 0.2 1.22 -1.07 -1.21 -Br -CI 2.1 0.7 0.2 2.2 0.5 0.2 -OAr 1.21 -0.60 -1.00 -OH 2.3 0.3 0.1 -O(C=O)R –NH, -NHR, –NR, -NH(C=O)R 2.11 -0.35 -0.64 -OR 2.1 0.3 0.1 0.80 -1.26 1.21 -OAr -O(C=OR -O(C=O)Ar -NH, -NH(C=O)R 2.8 0.5 0.3 2.8 0.5 0.1 2.08 -0.57 -0.72 3.1 0.5 0.2 1.5 0.2 0.1 a. There may be small differences in the chemical-shift values calculated from this table and those measured from individual spectra. 2.1 0.3 0.1 -NH(C=O)Ar 2.3 0.4 0.1 Page 1 of 1 O words English (United States) Focus + 140% lili
AutoSave OFF Document1 Home Insert Draw Design Layout References Mailings Review View Acrobat Tell me Share Comments Calibri (Bo... A A Аa v E v E - E v 12 >T A Styles Styles Pane Dictate Sensitivity Create and Share Adobe PDF Paste A v Request Signatures U v ab x, x -CH, -CH(CH,), -CH,CI -CH-CH, -CH=CHAr -0.18 -0.11 -0.21 -0,14 0.02 -0.08 --0.20 -0.01 Typical proton-proton coupling constants -0.04 TABLE 22.6 -0.12 -0.11 0.04 -0.04 0.14 -0.02 Arrangment of protons J(Hz) Arrangement protons J(Hz) Arrangement of protons J(Hz) -CH=CHCO,H -CH=CH(C=O)Ar 0.19 0.04 0.05 0.28 0.06 0.05 (Continued) 10 to 16 O to 3 Free rotation H. -H 8 to 13 11 to 14 12 to 18 H r Magnetic Resonance Spectroscopy 371 Anti H. Group ortho meta para -Ar 0.23 0.07 -0.02 2 to 4 8 to 13 6 to 12 -(C=O)H -(C-O)R 0.53 0.18 0.28 0.60 0.10 0.20 Gauche H. -(C=O)Ar 0.45 0.12 0.23 -(C-O)CH=CHA. -(C=O)OCH, -(C=0)OCH,CH, (C=O)OH -(C=0)CI -(C=O)NH, -CEN -F 0.67 0.68 0.14 0.21 0.08 0.19 0.69 0.06 0.17 0.77 0.11 0.25 6 to 9 2 to 6 4 to 10 0.76 0.16 0.33 0.46 0.09 0.17 0.29 0.12 0.25 -0.32 -0.05 -0.25 -0.13 -0.08 -CI -0.02 -0.07 -Br 0.13 -0.13 1 to 3 2 to 5 0.5 to 2 -0.43 -0.41 -OH -0.53 -0.14 -OR -0.45 -0.07 -OAr -0.36 -0.04 -0.28 -O(C=O)R -O(C=O)Ar -0.27 0.02 -0.13 -0.14 -0.71 -0.68 0.07 -0.09 -NH, -N(CH,), -NH(C=O)R -NO, -0.22 -0.62 O to 1 -0.15 -0.73 H. H. 0.14 -0.07 -0.27 0.87 0.20 0.35 a. Base value is the measured chemical shift of benzene in CDCI, (1% solution). Additive Parameters for Predicting NMR Chemical Shifts of Vinyl Protons in CDCI,a TABLE 22. 5 cis H Additive parameters for predicting NMR chemical shifts of alkyl protons in CDCI, TABLE 22.3 Base values trans gem Methyl Methylene Methine 0.9 ppm 1.2 ppm 1.5 ppm Base value 5.28 ppm Group (Y) Alpha (a) substituent Beta (B) substituent Gamma (y) substituent Group gem cis trans -R 0.45 -0.22 -0.28 H- -Y H- -Y H -0.01 -CH=CH, –CH,OH -CH,X (X=F, CI, Br) {C=O)OH -(C=O)OR 1.26 0.08 -R 0.0 0.0 0.64 -0.02 -0.04 0.0 -0.01 -C=C -C=C-Ar -C=C(C=O)OR -C=C-R 0.8 0.2 0.1 0.70 -0.11 0.9 0.1 0.0 0.71 0.55 1.0 0.3 0.1 0.97 1.41 0.9 0.3 0.1 0.80 1.18 -C=C-Ar 1.2 0.4 0.2 -(C=O)H -(C=0)R -Ar 1.4 0.4 0.1 1.02 0.95 1.17 -(C=O)OH -(C=O)OR -(C=O)H -(C=O)R -(C=O)An -(C=O)NH, -(C=O)CI -CEN 1.1 0.3 0.3 0.1 1.10 1.12 1.13 0.87 1.1 0.1 -(C=0)Ar -Ar 1.1 0.4 0.1 1.82 0.63 1.2 0.3 0.0 1.38 0.36 -0.07 1.7 0.3 0.1 0.45 0.18 -Br 1.07 0.55 1.0 0.3 0.1 -CI -OR 1.8 0.4 0.1 1.08 0.13 1.1 0.4 0.2 1.22 -1.07 -1.21 -Br -CI 2.1 0.7 0.2 2.2 0.5 0.2 -OAr 1.21 -0.60 -1.00 -OH 2.3 0.3 0.1 -O(C=O)R –NH, -NHR, –NR, -NH(C=O)R 2.11 -0.35 -0.64 -OR 2.1 0.3 0.1 0.80 -1.26 1.21 -OAr -O(C=OR -O(C=O)Ar -NH, -NH(C=O)R 2.8 0.5 0.3 2.8 0.5 0.1 2.08 -0.57 -0.72 3.1 0.5 0.2 1.5 0.2 0.1 a. There may be small differences in the chemical-shift values calculated from this table and those measured from individual spectra. 2.1 0.3 0.1 -NH(C=O)Ar 2.3 0.4 0.1 Page 1 of 1 O words English (United States) Focus + 140% lili
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL3: Carbon (13c) Nmr Spectroscopy
Section: Chapter Questions
Problem 8E
Related questions
Question
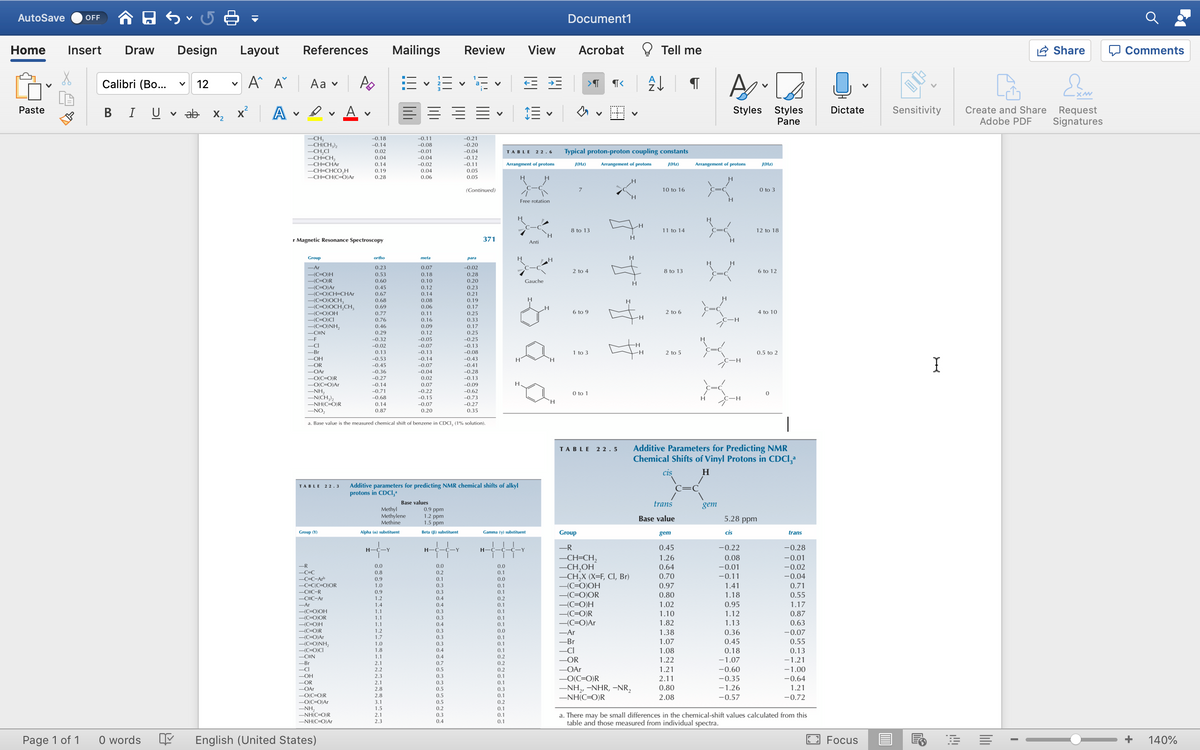
predict the shift for the carbon indicated by the arrows please.
USE NMR SPECTRUM TABLE please.

Transcribed Image Text:AutoSave
OFF
Document1
Home
Insert
Draw
Design
Layout
References
Mailings
Review
View
Acrobat
Tell me
Share
Comments
Calibri (Bo...
A A
Аa v
E v E - E v
12
>T
A
Styles Styles
Pane
Dictate
Sensitivity
Create and Share
Adobe PDF
Paste
A v
Request
Signatures
U
v ab x, x
-CH,
-CH(CH,),
-CH,CI
-CH-CH,
-CH=CHAr
-0.18
-0.11
-0.21
-0,14
0.02
-0.08
--0.20
-0.01
Typical proton-proton coupling constants
-0.04
TABLE 22.6
-0.12
-0.11
0.04
-0.04
0.14
-0.02
Arrangment of protons
J(Hz)
Arrangement
protons
J(Hz)
Arrangement of protons
J(Hz)
-CH=CHCO,H
-CH=CH(C=O)Ar
0.19
0.04
0.05
0.28
0.06
0.05
(Continued)
10 to 16
O to 3
Free rotation
H.
-H
8 to 13
11 to 14
12 to 18
H
r Magnetic Resonance Spectroscopy
371
Anti
H.
Group
ortho
meta
para
-Ar
0.23
0.07
-0.02
2 to 4
8 to 13
6 to 12
-(C=O)H
-(C-O)R
0.53
0.18
0.28
0.60
0.10
0.20
Gauche
H.
-(C=O)Ar
0.45
0.12
0.23
-(C-O)CH=CHA.
-(C=O)OCH,
-(C=0)OCH,CH,
(C=O)OH
-(C=0)CI
-(C=O)NH,
-CEN
-F
0.67
0.68
0.14
0.21
0.08
0.19
0.69
0.06
0.17
0.77
0.11
0.25
6 to 9
2 to 6
4 to 10
0.76
0.16
0.33
0.46
0.09
0.17
0.29
0.12
0.25
-0.32
-0.05
-0.25
-0.13
-0.08
-CI
-0.02
-0.07
-Br
0.13
-0.13
1 to 3
2 to 5
0.5 to 2
-0.43
-0.41
-OH
-0.53
-0.14
-OR
-0.45
-0.07
-OAr
-0.36
-0.04
-0.28
-O(C=O)R
-O(C=O)Ar
-0.27
0.02
-0.13
-0.14
-0.71
-0.68
0.07
-0.09
-NH,
-N(CH,),
-NH(C=O)R
-NO,
-0.22
-0.62
O to 1
-0.15
-0.73
H.
H.
0.14
-0.07
-0.27
0.87
0.20
0.35
a. Base value is the measured chemical shift of benzene in CDCI, (1% solution).
Additive Parameters for Predicting NMR
Chemical Shifts of Vinyl Protons in CDCI,a
TABLE 22. 5
cis
H
Additive parameters for predicting NMR chemical shifts of alkyl
protons in CDCI,
TABLE 22.3
Base values
trans
gem
Methyl
Methylene
Methine
0.9 ppm
1.2 ppm
1.5 ppm
Base value
5.28 ppm
Group (Y)
Alpha (a) substituent
Beta (B) substituent
Gamma (y) substituent
Group
gem
cis
trans
-R
0.45
-0.22
-0.28
H-
-Y
H-
-Y
H
-0.01
-CH=CH,
–CH,OH
-CH,X (X=F, CI, Br)
{C=O)OH
-(C=O)OR
1.26
0.08
-R
0.0
0.0
0.64
-0.02
-0.04
0.0
-0.01
-C=C
-C=C-Ar
-C=C(C=O)OR
-C=C-R
0.8
0.2
0.1
0.70
-0.11
0.9
0.1
0.0
0.71
0.55
1.0
0.3
0.1
0.97
1.41
0.9
0.3
0.1
0.80
1.18
-C=C-Ar
1.2
0.4
0.2
-(C=O)H
-(C=0)R
-Ar
1.4
0.4
0.1
1.02
0.95
1.17
-(C=O)OH
-(C=O)OR
-(C=O)H
-(C=O)R
-(C=O)An
-(C=O)NH,
-(C=O)CI
-CEN
1.1
0.3
0.3
0.1
1.10
1.12
1.13
0.87
1.1
0.1
-(C=0)Ar
-Ar
1.1
0.4
0.1
1.82
0.63
1.2
0.3
0.0
1.38
0.36
-0.07
1.7
0.3
0.1
0.45
0.18
-Br
1.07
0.55
1.0
0.3
0.1
-CI
-OR
1.8
0.4
0.1
1.08
0.13
1.1
0.4
0.2
1.22
-1.07
-1.21
-Br
-CI
2.1
0.7
0.2
2.2
0.5
0.2
-OAr
1.21
-0.60
-1.00
-OH
2.3
0.3
0.1
-O(C=O)R
–NH, -NHR, –NR,
-NH(C=O)R
2.11
-0.35
-0.64
-OR
2.1
0.3
0.1
0.80
-1.26
1.21
-OAr
-O(C=OR
-O(C=O)Ar
-NH,
-NH(C=O)R
2.8
0.5
0.3
2.8
0.5
0.1
2.08
-0.57
-0.72
3.1
0.5
0.2
1.5
0.2
0.1
a. There may be small differences in the chemical-shift values calculated from this
table and those measured from individual spectra.
2.1
0.3
0.1
-NH(C=O)Ar
2.3
0.4
0.1
Page 1 of 1
O words
English (United States)
Focus
+
140%
lili
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,