Practice 1.3 Microscale of volumes of solutions with different densities. For this exercise we will simulate the preparation of a reaction in a 0.5ml tube, using a micropipette with a range 1-20 ul. The idea is to create a "microscale" of volumes of colored solutions with different densities. 1. Use the table below to add the volumes in the tubes. Calculate the volume you need where they appear the X. Solución 3 (5%) Solución 1 (50%) 10 μι X = Solución 2 Solución 4 Volumen (25%) 8 ul 10 ul (0%) Total Tubo A 20 μι 20 ul Tubo B 2 ul
Practice 1.3 Microscale of volumes of solutions with different densities. For this exercise we will simulate the preparation of a reaction in a 0.5ml tube, using a micropipette with a range 1-20 ul. The idea is to create a "microscale" of volumes of colored solutions with different densities. 1. Use the table below to add the volumes in the tubes. Calculate the volume you need where they appear the X. Solución 3 (5%) Solución 1 (50%) 10 μι X = Solución 2 Solución 4 Volumen (25%) 8 ul 10 ul (0%) Total Tubo A 20 μι 20 ul Tubo B 2 ul
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.95PAE: 2.95 Engineers who design bicycle frames are familiar with the densities of aluminium (2.699 g/cm3),...
Related questions
Question
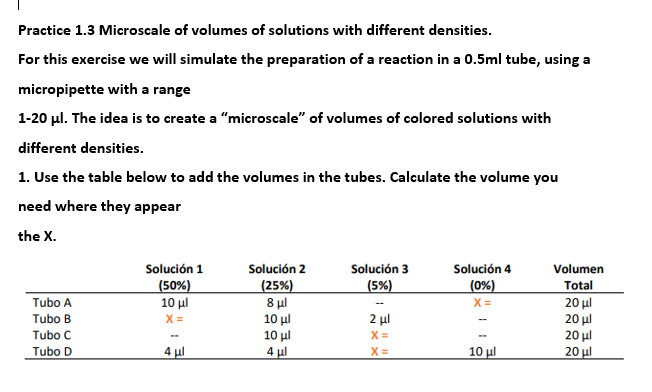
Transcribed Image Text:Practice 1.3 Microscale of volumes of solutions with different densities.
For this exercise we will simulate the preparation of a reaction in a 0.5ml tube, using a
micropipette with a range
1-20 ul. The idea is to create a "microscale" of volumes of colored solutions with
different densities.
1. Use the table below to add the volumes in the tubes. Calculate the volume you
need where they appear
the X.
Solución 3
(5%)
Solución 4
(0%)
Solución 1
Solución 2
Volumen
(50%)
10 μ
|(25%)
8 ul
10 μι
Total
20 μ
20 μί
20 µl
20 ul
Tubo A
X =
Tubo B
X =
2 µl
X =
X =
Tubo C
10μ
4 µl
Tubo D
4 ul
10 μΙ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning