PRACTICE EXAMPLE A: A 22.3 g sample of acetone (see the model here) is dissolved in enough water to produce 1.25 L of solution. What is the molarity of acetone in this solution? PRACTICE EXAMPLE B: If 15.0 mL of acetic acid, CH3COOH (d = 1.048 g/mL), is dissolved in enough water to produce 500.0 mL of solution, then what is the molarity of acetic acid in the solution?
PRACTICE EXAMPLE A: A 22.3 g sample of acetone (see the model here) is dissolved in enough water to produce 1.25 L of solution. What is the molarity of acetone in this solution? PRACTICE EXAMPLE B: If 15.0 mL of acetic acid, CH3COOH (d = 1.048 g/mL), is dissolved in enough water to produce 500.0 mL of solution, then what is the molarity of acetic acid in the solution?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.104QE: A 10.00-mL sample of a 24.00% solution of ammonium bromide (NH4Br) requires 23.41 mL of 1.200 molar...
Related questions
Question
PRACTICE EXAMPLE A: A 22.3 g sample of acetone (see the model here) is dissolved in enough water to produce 1.25 L of solution. What is the molarity of acetone in this solution? PRACTICE EXAMPLE B: If 15.0 mL of acetic acid, CH3COOH (d = 1.048 g/mL), is dissolved in enough water to produce 500.0 mL of solution, then what is the molarity of acetic acid in the solution?

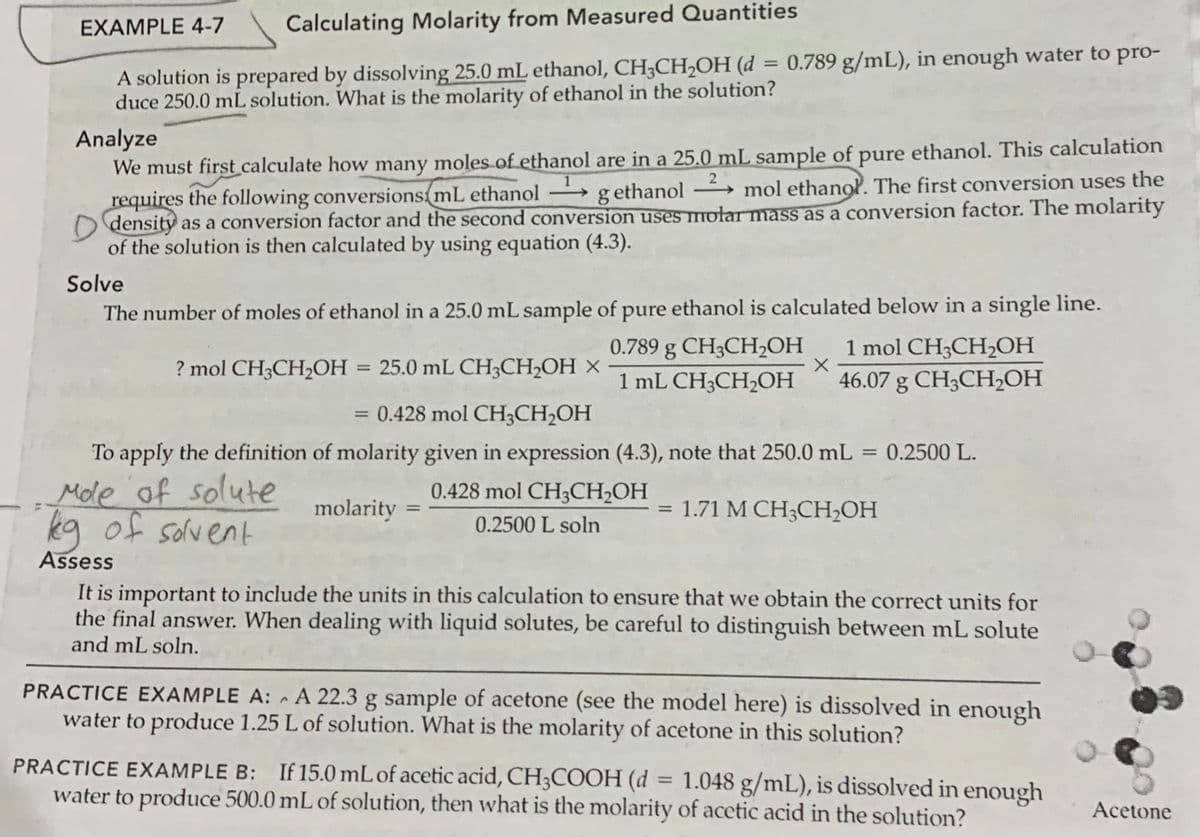
Transcribed Image Text:EXAMPLE 4-7
Calculating Molarity from Measured Quantities
= 0.789 g/mL), in enough water to pro-
A solution is prepared by dissolving 25.0 mL ethanol, CH3CH2OH (d
duce 250.0 mL solution. What is the molarity of ethanol in the solution?
Analyze
We must first calculate how many moles of ethanol are in a 25.0 mL sample of pure ethanol. This calculation
requires the following conversions mL ethanol
O density as a conversion factor and the second conversion uses mołar mass as a conversion factor. The molarity
of the solution is then calculated by using equation (4.3).
2
g ethanol → mol ethanol. The first conversion uses the
-
Solve
The number of moles of ethanol in a 25.0 mL sample of pure ethanol is calculated below in a single line.
1 mol CH3CH2OH
46.07 g CH3CH,OH
0.789 g CH3CH,OH
? mol CH3CH2OH
25.0 mL CH3CH2OH ×
%3D
1 mL CH3CH2OН
= 0.428 mol CH3CH2OH
%3D
To apply the definition of molarity given in expression (4.3), note that 250.0 mL = 0.2500 L.
%3D
Mole of solute
kg of solvent
0.428 mol CH3CH2OH
molarity
= 1.71 M CH;CH2OH
0.2500 L soln
Assess
It is important to include the units in this calculation to ensure that we obtain the correct units for
the final answer. When dealing with liquid solutes, be careful to distinguish between mL solute
and mL soln.
PRACTICE EXAMPLE A: - A 22.3 g sample of acetone (see the model here) is dissolved in enough
water to produce 1.25 L of solution. What is the molarity of acetone in this solution?
PRACTICE EXAMPLE B: If 15.0 mL of acetic acid, CH3COOH (d
water to produce 500.0 mL of solution, then what is the molarity of acetic acid in the solution?
1.048 g/mL), is dissolved in enough
%3D
Acetone
Transcribed Image Text:EXAMPLE 4-7
Calculating Molarity from Measured Quantities
= 0.789 g/mL), in enough water to pro-
A solution is prepared by dissolving 25.0 mL ethanol, CH3CH2OH (d
duce 250.0 mL solution. What is the molarity of ethanol in the solution?
Analyze
We must first calculate how many moles of ethanol are in a 25.0 mL sample of pure ethanol. This calculation
requires the following conversions mL ethanol
O density as a conversion factor and the second conversion uses molar mass as a conversion factor. The molarity
of the solution is then calculated by using equation (4.3).
g ethanol
mol ethanol. The first conversion uses the
-
Solve
The number of moles of ethanol in a 25.0 mL sample of pure ethanol is calculated below in a single line.
0.789 g CH3CH2OH
1 mol CH;CH2OH
? mol CH3CH2OH =
25.0 mL CH3CH2OH ×
%3D
1 mL CHCH2CН
46.07 g CH;CH,OH
= 0.428 mol CH3CH,OH
To apply the definition of molarity given in expression (4.3), note that 250.0 mL = 0.2500 L.
Mde of solute
kq of solvent
0.428 mol CH3CH2OH
molarity =
1.71 M CH3CH2OH
%3D
0.2500 L soln
Assess
It is important to include the units in this calculation to ensure that we obtain the correct units for
the final answer. When dealing with liquid solutes, be careful to distinguish between mL solute
and mL soln.
PRACTICE EXAMPLE A: A 22.3 g sample of acetone (see the model here) is dissolved in enough
water to produce 1.25 L of solution. What is the molarity of acetone in this solution?
PRACTICE EXAMPLE B: If 15.0 mL of acetic acid, CH;COOH (d
water to produce 500.0 mL of solution, then what is the molarity of acetic acid in the solution?
= 1.048 g/mL), is dissolved in enough
%3D
Acetone
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
