PRACTICE EXAMPLE A: potassium chlorate? How many moles of O₂ are produced from the decomposition of 1.76 moles of 2 KCIO3(s) →→→2 KCl(s) + 3 O₂(g) PRACTICE EXAMPLE B: How many moles of Ag are produced in the decomposition of 1.00 kg of silver oxide? 2 Ag₂O(s) 4 Ag(s) + O₂(g)
PRACTICE EXAMPLE A: potassium chlorate? How many moles of O₂ are produced from the decomposition of 1.76 moles of 2 KCIO3(s) →→→2 KCl(s) + 3 O₂(g) PRACTICE EXAMPLE B: How many moles of Ag are produced in the decomposition of 1.00 kg of silver oxide? 2 Ag₂O(s) 4 Ag(s) + O₂(g)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 33QAP: A lead ore, galena, consisting mainly of lead(II) sulfide, is the principal source of lead. To...
Related questions
Question
PRACTICE EXAMPLE A: potassium chlorate? How many moles of O₂ are produced from the decomposition of 1.76 moles of
2 KCIO3(s) →→→2 KCl(s) + 3 O₂(g)
PRACTICE EXAMPLE B: How many moles of Ag are produced in the decomposition of 1.00 kg of silver oxide?
2 Ag₂O(s) 4 Ag(s) + O₂(g)
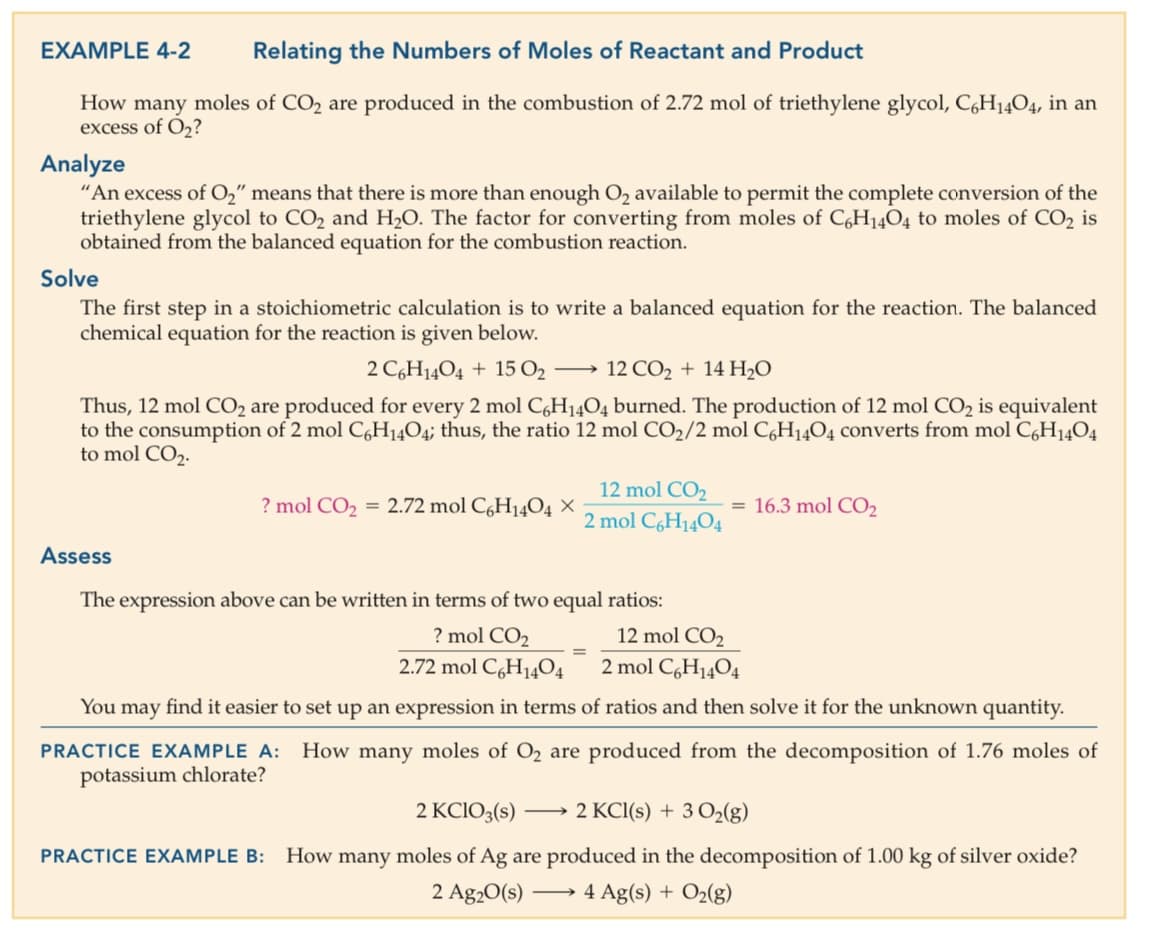
Transcribed Image Text:EXAMPLE 4-2
Relating the Numbers of Moles of Reactant and Product
How many moles of CO2 are produced in the combustion of 2.72 mol of triethylene glycol, C,H1404, in an
excess of O,?
Analyze
"An excess of O," means that there is more than enough O2 available to permit the complete conversion of the
triethylene glycol to CO2 and H20. The factor for converting from moles of C,H14O4 to moles of CO2 is
obtained from the balanced equation for the combustion reaction.
Solve
The first step in a stoichiometric calculation is to write a balanced equation for the reaction. The balanced
chemical equation for the reaction is given below.
2 C,H14O4 + 15 O2 →
12 CO2 + 14 H20
Thus, 12 mol CO2 are produced for every 2 mol C,H14O4 burned. The production of 12 mol CO2 is equivalent
to the consumption of 2 mol C,H14O4; thus, the ratio 12 mol CO2/2 mol C,H1404 converts from mol C,H1404
to mol CO2.
12 mol CO2
? mol CO2 = 2.72 mol CgH14O4 ×
= 16.3 mol CO2
2 mol C6H1404
Assess
The expression above can be written in terms of two equal ratios:
? mol CO2
12 mol CO2
2.72 mol C,H14O4
2 mol C,H1404
You may find it easier to set up an expression in terms of ratios and then solve it for the unknown quantity.
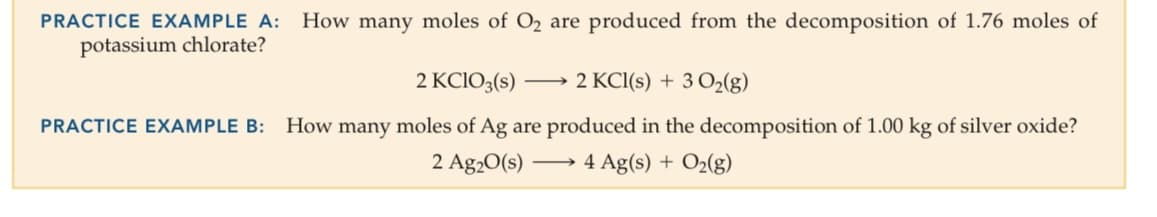
PRACTICE EXAMPLE A: How many moles of O2 are produced from the decomposition of 1.76 moles of
potassium chlorate?
2 KCIO3(s)
2 KCI(s) + 3 O2(g)
-
PRACTICE EXAMPLE B:
How many moles of Ag are produced in the decomposition of 1.00 kg of silver oxide?
2 Ag20(s)
4 Ag(s) + O2(g)
>
Transcribed Image Text:PRACTICE EXAMPLE A:
How many moles of O2 are produced from the decomposition of 1.76 moles of
potassium chlorate?
2 KCIO3(s)
2 KCI(s) + 3 O2(g)
PRACTICE EXAMPLE B:
How many moles of Ag are produced in the decomposition of 1.00 kg of silver oxide?
2 Ag20(s)
4 Ag(s) + O2(g)
-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning