Predict the rate of the reaction in the following. Assume that the substances below are reactants for different reactions. Indicate if the reaction will be slower, faster, or have the same rate. Normal condition Changed condition Factor Reaction rate d) A cube of sugar powdered sugar Surface area Faster e) 5 sampalok cubes 1 sampalok cube Concentration Slower 0 52 g of NaOH 65 g of NAOH Concentration Faster 9) 3.68 x 10 atoms of Li 9.25 x 10 atoms Li Concentration Slower W 500 K 45% Barium 600K, 4.5% Barium Temperature Faster 48 g powdered zinc 48 g zinc pellets Surface area Slower ) Starch + water Starch + water + amylase Catalyst Faster
Predict the rate of the reaction in the following. Assume that the substances below are reactants for different reactions. Indicate if the reaction will be slower, faster, or have the same rate. Normal condition Changed condition Factor Reaction rate d) A cube of sugar powdered sugar Surface area Faster e) 5 sampalok cubes 1 sampalok cube Concentration Slower 0 52 g of NaOH 65 g of NAOH Concentration Faster 9) 3.68 x 10 atoms of Li 9.25 x 10 atoms Li Concentration Slower W 500 K 45% Barium 600K, 4.5% Barium Temperature Faster 48 g powdered zinc 48 g zinc pellets Surface area Slower ) Starch + water Starch + water + amylase Catalyst Faster
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 1RQ: Characterize a system at chemical equilibrium with respect to each of the following a. the rates of...
Related questions
Question
Please I don't need an explanation. Just the answer.
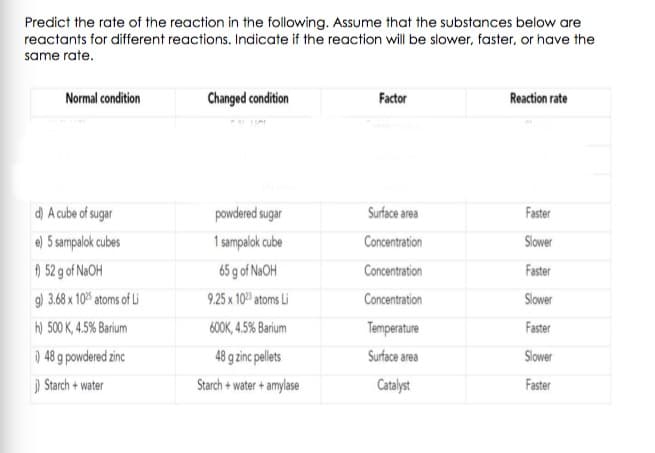
Transcribed Image Text:Predict the rate of the reaction in the following. Assume that the substances below are
reactants for different reactions. Indicate if the reaction will be slower, faster, or have the
same rate.
Normal condition
Changed condition
Factor
Reaction rate
d) A cube of sugar
powdered sugar
Surface area
Faster
e) 5 sampalok cubes
1 sampalok cube
Concentration
Slower
) 52 g of NaOH
65 g of N2OH
Concentration
Faster
9) 3.68 x 10% atoms of Li
9.25 x 10 atoms Li
Concentration
Slower
h) 500 K, 4.5% Barium
600K, 4.5% Barium
Temperature
Faster
) 48 g powdered zinc
48 g zinc pellets
Surface area
Slower
) Starch + water
Starch + water + amylase
Catalyst
Faster
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning