Predict whether the effects of molecular volume and of intermolecular attractions become more or less significant when the following changes are imposed: (a) A gas is expanded to a larger volume at constant temperature. Intermolecular attractions are significant, and molecular sizes are significant. (b) More gas is introduced into a container of constant volume at constant temperature. Intermolecular attractions are significant, and molecular sizes are significant. more (c) The temperature of a gas increases at constant pressure. less Intermolecular attractions are significant, and molecular sizes are v significant.
Predict whether the effects of molecular volume and of intermolecular attractions become more or less significant when the following changes are imposed: (a) A gas is expanded to a larger volume at constant temperature. Intermolecular attractions are significant, and molecular sizes are significant. (b) More gas is introduced into a container of constant volume at constant temperature. Intermolecular attractions are significant, and molecular sizes are significant. more (c) The temperature of a gas increases at constant pressure. less Intermolecular attractions are significant, and molecular sizes are v significant.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 104AP
Related questions
Question
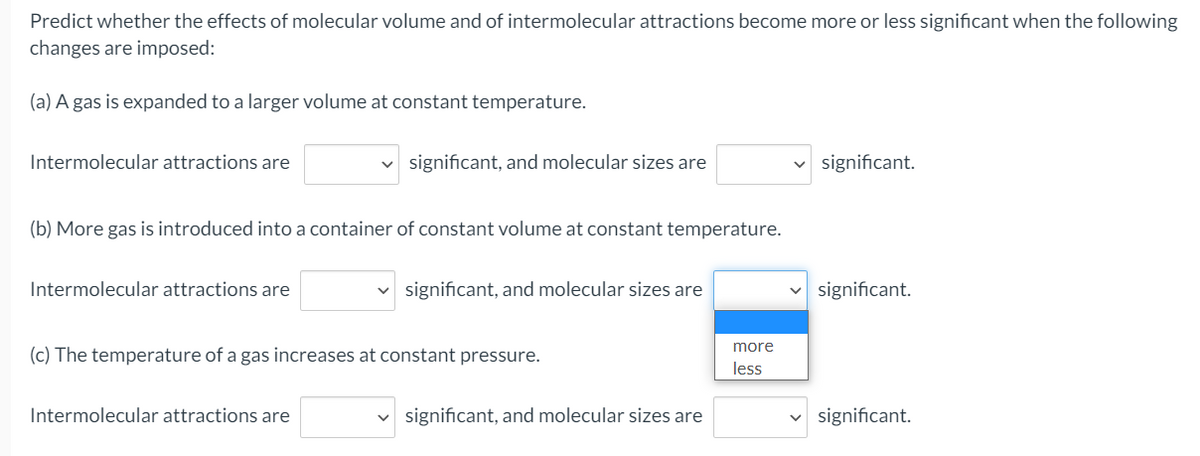
Transcribed Image Text:Predict whether the effects of molecular volume and of intermolecular attractions become more or less significant when the following
changes are imposed:
(a) A gas is expanded to a larger volume at constant temperature.
Intermolecular attractions are
significant, and molecular sizes are
v significant.
(b) More gas is introduced into a container of constant volume at constant temperature.
Intermolecular attractions are
significant, and molecular sizes are
significant.
more
(c) The temperature of a gas increases at constant pressure.
less
Intermolecular attractions are
significant, and molecular sizes are
significant.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning