presented by Macmillan Learning The quantity of antimony in a sample can be determined by an oxidation-reduction titration with an oxidizing agent. A 6.47 g sample of stibnite, an ore of antimony, is dissolved in hot, concentrated HCl(aq) and passed over a reducing agent so that all the antimony is in the form Sb*(aq). The Sb*(aq) is completely oxidized by 35.0 mL of a 0.105 M aqueous solution of KBRO, (aq). The unbalanced equation for the reaction is BrO, (aq) + Sb³*(aq) Br (aq) + Sb³* (aq) (unbalanced) Calculate the amount of antimony in the sample and its percentage in the ore. mass of antimony: percentage of antimony: Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemsitry | Publisher: University Science Books
presented by Macmillan Learning The quantity of antimony in a sample can be determined by an oxidation-reduction titration with an oxidizing agent. A 6.47 g sample of stibnite, an ore of antimony, is dissolved in hot, concentrated HCl(aq) and passed over a reducing agent so that all the antimony is in the form Sb*(aq). The Sb*(aq) is completely oxidized by 35.0 mL of a 0.105 M aqueous solution of KBRO, (aq). The unbalanced equation for the reaction is BrO, (aq) + Sb³*(aq) Br (aq) + Sb³* (aq) (unbalanced) Calculate the amount of antimony in the sample and its percentage in the ore. mass of antimony: percentage of antimony: Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemsitry | Publisher: University Science Books
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.103QE
Related questions
Question
9
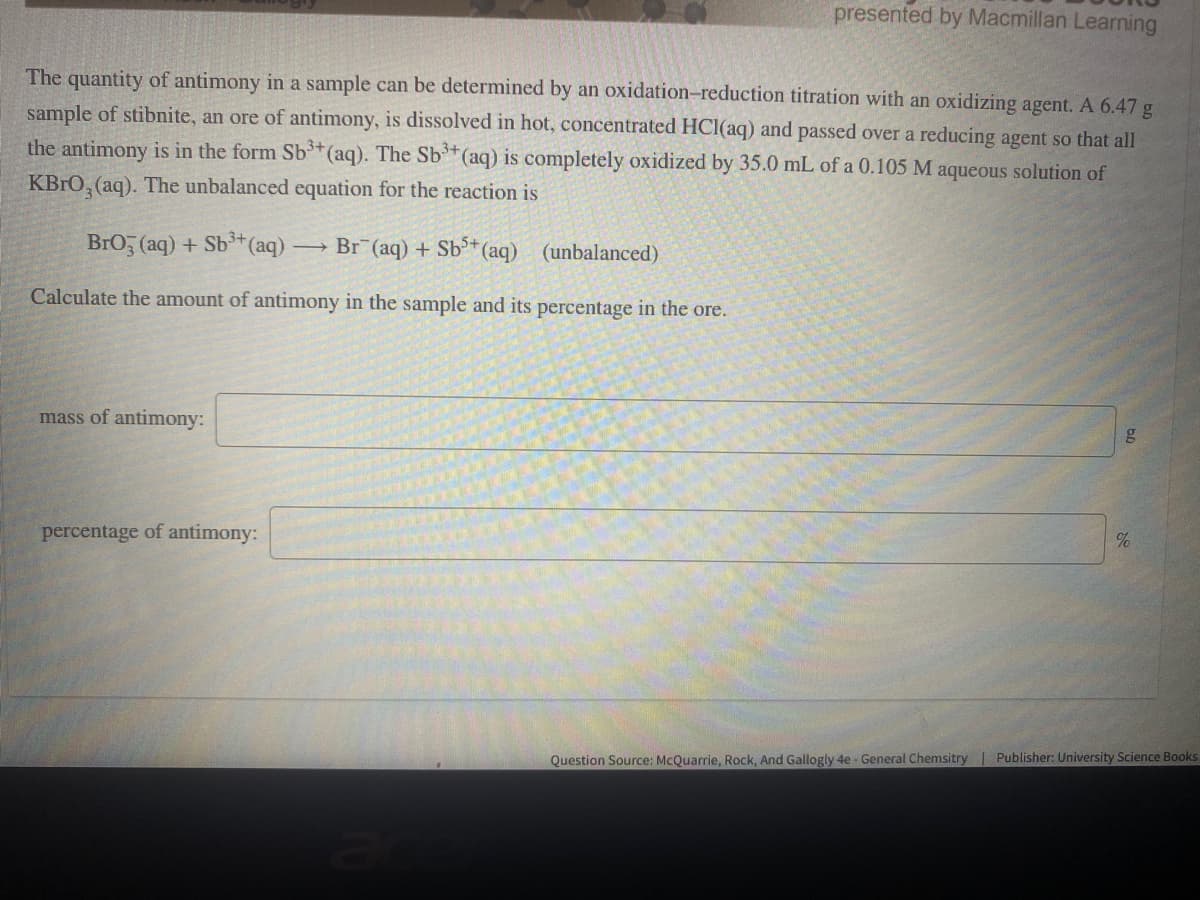
Transcribed Image Text:presented by Macmillan Learning
The quantity of antimony in a sample can be determined by an oxidation-reduction titration with an oxidizing agent. A 6.47 g
sample of stibnite, an ore of antimony, is dissolved in hot, concentrated HCI(aq) and passed over a reducing agent so that all
the antimony is in the form Sb**(aq). The Sb* (aq) is completely oxidized by 35.0 mL of a 0.105 M aqueous solution of
KBRO, (aq). The unbalanced equation for the reaction is
BrO, (aq) + Sb³*(aq)
Br (aq) + Sb³+ (aq)
(unbalanced)
Calculate the amount of antimony in the sample and its percentage in the ore.
mass of antimony:
percentage of antimony:
Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemsitry | Publisher: University Science Books
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
