Problem 11 K 11 of Review I Constants I Periodic Butane, C4H10, reacts with oxygen, O2, to form water, H2 O, and carbon dioxide, CO2, as shown in the following chemical equation: Submit Previous Answers 2C4H10(g) +1302 (g)+10H2O(g) +8CO2 (g) The coefficients in this equation represent mole ratios. Notice that the coefficient for water (10) is five times that of butane (2). Thus, the number of moles of water produced is five times the number of moles of butane that react. Correct Part C Also, notice that the coefficient for butane (2) is one- fourth the coefficient of carbon dioxide (8). Thus, the number of moles of butane that react is one-fourth the number of moles of carbon dioxide that you produce. But be careful! If you are given the mass of a compound, you must first convert to moles before applying these ratios. Calculate the mass of butane needed to produce 61.4 g of carbon dioxide. Express your answer to three significant figures and include the appropriate units. View Available Hint(s) ? HA Units Value Submit P Pearson Privacy Policy | Permissions | Contact Us Copyright 2019 Pearson Education Inc. All rights reserved.| Terms of Use t
Problem 11 K 11 of Review I Constants I Periodic Butane, C4H10, reacts with oxygen, O2, to form water, H2 O, and carbon dioxide, CO2, as shown in the following chemical equation: Submit Previous Answers 2C4H10(g) +1302 (g)+10H2O(g) +8CO2 (g) The coefficients in this equation represent mole ratios. Notice that the coefficient for water (10) is five times that of butane (2). Thus, the number of moles of water produced is five times the number of moles of butane that react. Correct Part C Also, notice that the coefficient for butane (2) is one- fourth the coefficient of carbon dioxide (8). Thus, the number of moles of butane that react is one-fourth the number of moles of carbon dioxide that you produce. But be careful! If you are given the mass of a compound, you must first convert to moles before applying these ratios. Calculate the mass of butane needed to produce 61.4 g of carbon dioxide. Express your answer to three significant figures and include the appropriate units. View Available Hint(s) ? HA Units Value Submit P Pearson Privacy Policy | Permissions | Contact Us Copyright 2019 Pearson Education Inc. All rights reserved.| Terms of Use t
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section5.2: Empirical Gas Laws
Problem 5.2CC
Related questions
Question
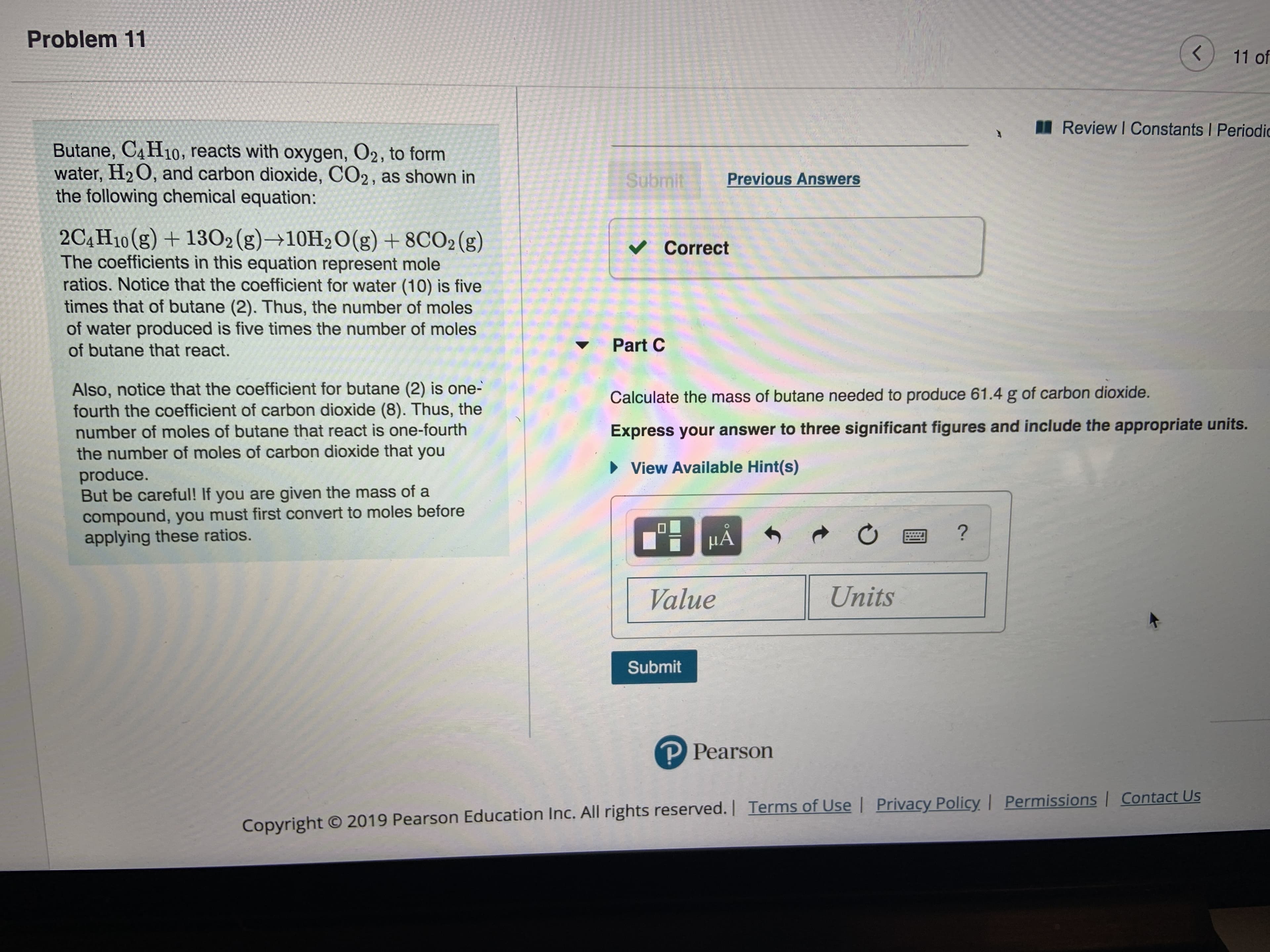
Transcribed Image Text:Problem 11
K
11 of
Review I Constants I Periodic
Butane, C4H10, reacts with oxygen, O2, to form
water, H2 O, and carbon dioxide, CO2, as shown in
the following chemical equation:
Submit
Previous Answers
2C4H10(g) +1302 (g)+10H2O(g) +8CO2 (g)
The coefficients in this equation represent mole
ratios. Notice that the coefficient for water (10) is five
times that of butane (2). Thus, the number of moles
of water produced is five times the number of moles
of butane that react.
Correct
Part C
Also, notice that the coefficient for butane (2) is one-
fourth the coefficient of carbon dioxide (8). Thus, the
number of moles of butane that react is one-fourth
the number of moles of carbon dioxide that you
produce.
But be careful! If you are given the mass of a
compound, you must first convert to moles before
applying these ratios.
Calculate the mass of butane needed to produce 61.4 g of carbon dioxide.
Express your answer to three significant figures and include the appropriate units.
View Available Hint(s)
?
HA
Units
Value
Submit
P Pearson
Privacy Policy | Permissions | Contact Us
Copyright 2019 Pearson Education Inc. All rights reserved.| Terms of Use
t
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning