Problem 16.77 Ephedrine, a central nervous system stimulant, is used in nasal sprays as a decongestant. This compound is a weak organic base: C10H15ON(aq) + H₂O(1) C₁0H15ONH¹ (aq) + OH (aq) A 0.035 M solution of ephedrine has a pH of 11.33. ▼ Part A What is the equilibrium concentration of C10H15 ON? Express your answer using two significant figures. ΠΙΑΣΦ [C10H15 ON] = Submit Part B What is the equilibrium concentration of CIOHISONH¹? Express your answer using two significant figures. |CHısONH | Submit Request Answer Part C TOHT= VD] ΑΣΦ Request Answer 3 What is the equilibrium concentration of OH? Express your answer using two significant figures. V. ΑΣΦ Pearson ? ? M ? M M 26 of 43 Review | Constants | Periodic Table
Problem 16.77 Ephedrine, a central nervous system stimulant, is used in nasal sprays as a decongestant. This compound is a weak organic base: C10H15ON(aq) + H₂O(1) C₁0H15ONH¹ (aq) + OH (aq) A 0.035 M solution of ephedrine has a pH of 11.33. ▼ Part A What is the equilibrium concentration of C10H15 ON? Express your answer using two significant figures. ΠΙΑΣΦ [C10H15 ON] = Submit Part B What is the equilibrium concentration of CIOHISONH¹? Express your answer using two significant figures. |CHısONH | Submit Request Answer Part C TOHT= VD] ΑΣΦ Request Answer 3 What is the equilibrium concentration of OH? Express your answer using two significant figures. V. ΑΣΦ Pearson ? ? M ? M M 26 of 43 Review | Constants | Periodic Table
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 10RQ: For oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen...
Related questions
Question
![Problem 16.77
Ephedrine, a central nervous system stimulant, is used in nasal sprays as
a decongestant. This compound is a weak organic base:
C10H15 ON (aq) + H₂O(1) C10H15ONH+ (aq) + OH (aq)
A 0.035 M solution fephedrine has a pH of 11.33.
Part A
What is the equilibrium concentration of C10H15ON?
Express your answer using two significant figures.
VΠΙΑΣΦ
[C10H15 ON] =
Submit
Part B
What is the equilibrium concentration of C10H15ONH?
Express your answer using two significant figures.
|VL]ΑΣΦ
[CıoHısONH'|=
Submit
Request Answer
Part C
[OH 1 =
Request Answer
What is the equilibrium concentration of OH ?
Express your answer using two significant figures.
ΠΑΣΦ
Pearson
?
M
M
M
26 of 43
Review | Constants | Periodic Table
>](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F7ba769ef-ff41-4f27-8bf9-2761389b1d49%2Fb84fd1d0-0a59-42ae-97a5-439657954212%2F0robyah_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Problem 16.77
Ephedrine, a central nervous system stimulant, is used in nasal sprays as
a decongestant. This compound is a weak organic base:
C10H15 ON (aq) + H₂O(1) C10H15ONH+ (aq) + OH (aq)
A 0.035 M solution fephedrine has a pH of 11.33.
Part A
What is the equilibrium concentration of C10H15ON?
Express your answer using two significant figures.
VΠΙΑΣΦ
[C10H15 ON] =
Submit
Part B
What is the equilibrium concentration of C10H15ONH?
Express your answer using two significant figures.
|VL]ΑΣΦ
[CıoHısONH'|=
Submit
Request Answer
Part C
[OH 1 =
Request Answer
What is the equilibrium concentration of OH ?
Express your answer using two significant figures.
ΠΑΣΦ
Pearson
?
M
M
M
26 of 43
Review | Constants | Periodic Table
>
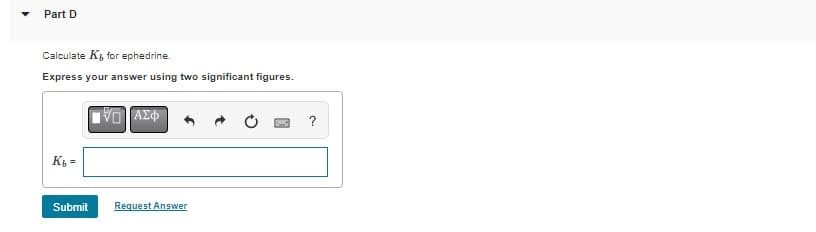
Transcribed Image Text:Part D
Calculate K, for ephedrine.
Express your answer using two significant figures.
Kb =
Submit
ΕΠΙ ΑΣΦ
Request Answer
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning