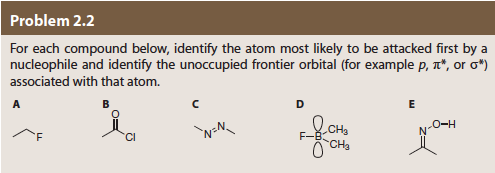
Problem 2.2 For each compound below, identify the atom most likely to be attacked first by a nucleophile and identify the unoccupied frontier orbital (for example p, T", or σ*) associated with that atom. Cl
Q: Example 4. exocyclic double bond for ring-C and also ring-A C A в exocyclic double bond for ring-C…
A:
Q: Below is the SN2 reaction between chlorocyclohexane and cyanide (CN-). Draw the missing curved arrow…
A: The SN2 reaction between chlorocyclohexane and cyanide (CN-) is given below,
Q: 4. [Short answer] What is the IUPAC name for the compound shown below?
A:
Q: Draw all significant resonance contributors for the following and label each contributor as major,…
A: The structure of all the resonance structures is given below.
Q: Compound A has a molecular formula of C,H0. What is the Unsaturation Number (UN) or Double-Bond…
A: To find out the structure of a compound whose molecular formula is given to be C6H14O. The NMR data…
Q: 1.78 (a) Add curved arrows to show how the starting material A is converted to the product B. (b)…
A: Since you have asked multiple question, we will solve the first question for you. If youwant any…
Q: 3 attempts left Check my work Which of the following species are conjugated? Select all that apply.
A:
Q: N- N.
A: There are three general conditions for a molecule to be Aromatic - The compound must be planar i.e.…
Q: I asked this question earlier, and I was given the wrong answer, please fix this and if possible,…
A: Anthracene is a solid polycyclic aromatic hydrocarbon [ formula C14H10]. It consists of three fused…
Q: a. HN02 H12504 b. draw the resonance C. Which resonance Structure is most stable ? would react…
A: Ketone group is an ElectronWithdrawing group and Electrophile Attach at meta position .
Q: 4. Answer the following question(s) concerning sulfathiazole, below. Furan 4(a) What is the…
A: Factors which explain the compound is aromatic is shown below: The molecule must be cyclic. The…
Q: Give the IUPAC name for each compound.
A: a. Please find below the IUPAC name along with the structure of the molecule. Since we have longest…
Q: Circle or write in the correct answer in the bold/underlined multiple choice below: 1. Among the…
A: We have to write in the correct answer in the bold/underlined multiple choice given as follows in…
Q: с.
A: Resonance structures are madeup via flow of electrons.
Q: References AEP PRACTICE the skill 17.13 For each of the following compounds determine which (if any)…
A: Hey, since there are multiple sub-part questions posted, we will answer first three questions. If…
Q: 5. Explain why A is much more stable than B? B NH
A: To explain why compound (A) is more stable than the compound (B)
Q: Every box should contain two structures. Be sure to include all lone pair electrons and nonzero…
A: In the above reaction, first the alkene will undergo electrophilic addition of proton with H3O+…
Q: Meet benzylpenicillin, a common antibiotic for bacterial infections. Answer the following parts…
A:
Q: For A-C draw each fisher projection with the -COH group on the 2 top OH но OH OH зе зде OH он OH OH…
A: A stereo formula can be represented in two dimensions using the Fischer projection or Fischer…
Q: 3. Which proton(blue) is more downfield? а. OMe H H2C 3b. Which proton is more upfield(red or blue)?…
A:
Q: Carbocation stability is important in reaction mechanisms involving alcohols. Based on the general…
A:
Q: Page 1 of 2 PRACTICE PROBLEMS 5A. For chains, draw Newman projections of all conformations around a…
A: Here we have to write Newman projection of the following given compounds.
Q: 4. Answer the following question(s) concerning sulfathiazole, below. Furan 4(a) What is the…
A:
Q: 2 Circle which of the following compounds are aromatic. Place a star inside which compounds are…
A:
Q: Label each C–C double bond as E or Z.
A: The E isomer has the two higher priority groups on the opposite sides. The Z isomer has the two…
Q: 1. Draw and Determine the hybridization of the carbon atom in (a) carbocation, (b) carbanion, and…
A: Hi, we are supposed to answer one question. To get the remaining questions solved please mention the…
Q: 3. [Short answer] What is the IUPAC name for the compound shown below?
A: The numbering of longest continuous carbon chain is given in such a way that the functional group…
Q: 4) Draw the most stable and least stable Newman projections of the following compound viewed along…
A: Newman projection involves representation of conformation of the structural formula of compound with…
Q: 1. NaNH2 2. Br
A: Acid-base reaction Nucleophilic bimolecu
Q: See the Attachment & solev the followings (a) Add curved arrows to show how the starting…
A: (a) Conversion of A to B
Q: Part A Rank the following compounds in order from most stable to least stable: Rank from most stable…
A:
Q: n statement is most correct for the compounds labeled A and B? norbornenes ompound B is more stable…
A: Whenever a double bond is present on the carbon which is making a bridge in norbornenes it leads a…
Q: Draw the curved arrows and the resulting resonance structure of meta-nitrobenzoate that shows a…
A:
Q: Q2 / Draw the following compounds so that they are optically active. A) Br-CH2-CH2…
A:
Q: Label attached C–C double bond as E or Z.
A: This compound is E alkene.
Q: 4) Assign E /Z designation to each double bond where applicable. a) b) N, NH Br c)
A: Stereoisomers are the molecules having same molecular formula but differ only on the spatial…
Q: Practice Problems Complete and balance each substitution reaction. 1. HOH + (CH3);CI 2. CH3CH2CH3 +…
A: 2CH3CH2CH3 + 2Cl2 + sunlight is a chain reaction and more then one substitution is takes place. This…
Q: Solvent choice: In analyzing dichloromethane, what are it's properties (low BP 50°C) A) low BP,…
A: Answer
Q: Compare picture I and II and choose: A = If the indicated property is greater in picture I than in…
A:
Q: Q4/ Label each compound as aromatic, nonaromatic, or antiaromatic. b) e) d)
A: Here I have mentioned the aromatic, non-aromatic, and anti-aromatic species Conditions for aromatic=…
Q: e) Circle the most basic nitrogen atom in the compound below. NH2
A: Since you have asked multiple question, we will solve the first question for you. If youwant any…
Q: Saquinavir (trade name Invirase) is a protease inhibitor, used to treat HIV(human immunodeficiency…
A: Given compound:
Q: I deu But weekly, each one containing a few questions covering material from the previous week's…
A: We have to draw curved arrows to shor the interconversion of given resonance structures
Q: Instructions: a. Identify how many pi electrons present in the compound. b. Identify the following…
A: A compound can be defined as a substance composed of many molecules that are made up of atoms of…
Q: 玩,唱 2 / 2 85% Q3. Provide IUPAC name for each of the following compounds a b Br Me с. d.
A:
Q: 1.78 (a) Add curved arrows to show how the starting material A is converted to the product B. (b)…
A:
Q: 2 points. 1. Which of the following is the CORRECT Keq expression for the reaction given below? * +…
A: We are given the following balanced chemical reaction; CH4(g) + H2O(g) <------------> CO(g) +…
Q: Draw the conjugate bases of pyrrole and cyclopentadiene. Explain whythe sp3 hybridized C—H bond of…
A: The conjugate bases of pyrrole and cyclopentadiene has to be drawn.
Q: Compound B: Η Η Η Η Η Η Η Η Η H—c- Η H=C=H Η Select Draw the skeletal structure of Compound B. Draw…
A: In skeletal structure or bond-line structure, each sigma bond between two C atoms is represented by…
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

- Compare picture I and II and choose: A = If the indicated property is greater in picture I than in II B = If the indicated property is less in picture I than in II C = If the indicated property is equal in both picture D = If no comparison can be made due to insufficient information nucleophilic character of -OHOchem help regarding tautomers and constitutional isomers in the attached image: Classify each of the 5 pairs as tautomers, constitutional isomers, or not related (different compounds) I'm thinking pair II is a tautomer, but I'm not sure if IV and V are also tautomer pair I and III I'm thinking is not related (different compounds) Not sure though - so please HELPplease need clear answer drawing resonance for each group
- Draw a stepwise mechanism for the reaction shown below. Use curved arrows to show all bonds broken and formed, draw the structure of all intermediates, and include any formal charges.Does +I effect help in resonance and -I effect reduce the effect of resonance? Answer Follow · 1 RequestDraw all significant resonance structures for the following molecules. For each, circle the most significant resonance contributor..
- Answer each part for the reaction below a. Draw in ALL missing lone pairs b. In the first two boxes provided, label the starting materials as acid and base (use the given pKa table if necessary) c. Draw appropriate curved arrows to complete the acid-base reaction d. Draw the corresponding products for the reaction e. Using pKa values determine which side the equilibrium will favor and redraw the equilibrium arrow in the box provided, or if it will not favor either side write “neither” in the boxDraw the line-angle structure of the following compounds if applicable. note: I need the answer immediately. I will send a good rate right away as well.draw a newman projection of b viewed from C-2 to C-3 according to first template, using newman projection to demonstarte the most stable confromation around C2-C3 bond according to 2nd template
- Not sure if (a.) is correct. But I need help understanding how to do (b.).OChem help First, can you see if I have the IUPAC name correct for the drawn compound... Second will you please help with questions B. and C. Thank youWrite the name for Cr(CO)5. Group of answer choices pentacarbonlychromium (V) pentacarbonlychromium (0) pentacarbonlychromate (II) pentacarbonlychromium (II) pentacarbonlychromium (III) pentacarbonlychromate (III) pentacarbonlychromate (0) pentacarbonlychromate (V)

