Problem 5 Consider an electron in the hydrogen atom. groundstate. The electron is initially in the b) After excitation, the electron relaxes into the 3d' configuration. Deter- mine the term symbols of the new electron configuration. For each term symbol (level) that you find, determine: • The magnitude of the total angular momentum. • The degeneracy of the level.
Problem 5 Consider an electron in the hydrogen atom. groundstate. The electron is initially in the b) After excitation, the electron relaxes into the 3d' configuration. Deter- mine the term symbols of the new electron configuration. For each term symbol (level) that you find, determine: • The magnitude of the total angular momentum. • The degeneracy of the level.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section11.10: Electron Configurations And The Periodic Table
Problem 11.3SC
Related questions
Question
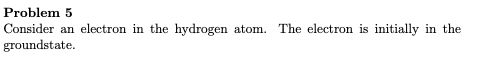
Transcribed Image Text:Problem 5
Consider an electron in the hydrogen atom.
groundstate.
The electron is initially in the

Transcribed Image Text:b) After excitation, the electron relaxes into the 3d' configuration. Deter-
mine the term symbols of the new electron configuration. For each term
symbol (level) that you find, determine:
• The magnitude of the total angular momentum.
• The degeneracy of the level.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning