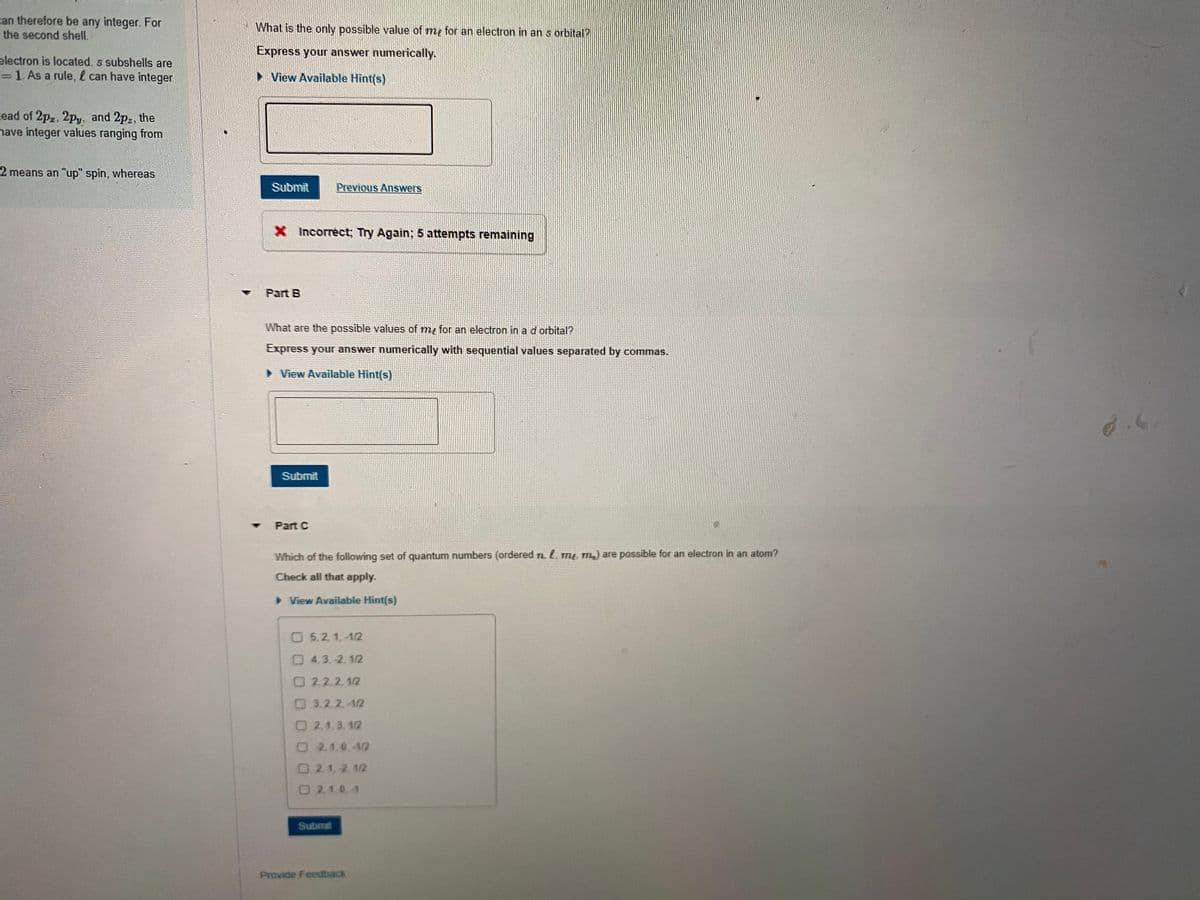
What is the only possible value of my for an electron in an s orbital? Express your answer numerically. > View Available Hint(s) Submit Previous Answers X Incorrect; Try Again; 5 attempts remaining
What is the only possible value of my for an electron in an s orbital? Express your answer numerically. > View Available Hint(s) Submit Previous Answers X Incorrect; Try Again; 5 attempts remaining
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 6P: Using Table 5.2, write down the mathematical expression for the 2px wave function for an...
Related questions
Question
Transcribed Image Text:can therefore be any integer. For
the second shell.
What is the only possible value of my for an electron in an s orbital?
Express your answer numerically.
electron is located. s subshells are
=1. As a rule, l can have integer
• View Available Hint(s)
cead of 2pz, 2py, and 2pz, the
nave integer values ranging from
2 means an "up" spin, whereas
Submit
Previous Answers
X Incorrect; Try Again; 5 attempts remaining
Part B
What are the possible values of my for an electron in ad orbital?
Express your answer numerically with sequential values separated by commas.
> View Available Hint(s)
Submit
Part C
Which of the following set of quantum numbers (ordered n. l. me m) are possible for an electron in an atom?
Check all that apply.
> View Available Hint(s)
O5.2, 1,-1/2
04,3.-2. 1/2
O2.2.2.1/2
O 3.2.2.-1/2
O2.1.3. 1/2
O2.1.0.-1/2
O2.1.-2. 1/2
O2.1.0.-1
Submit
Provide Feedback
Transcribed Image Text:Learning Goal:
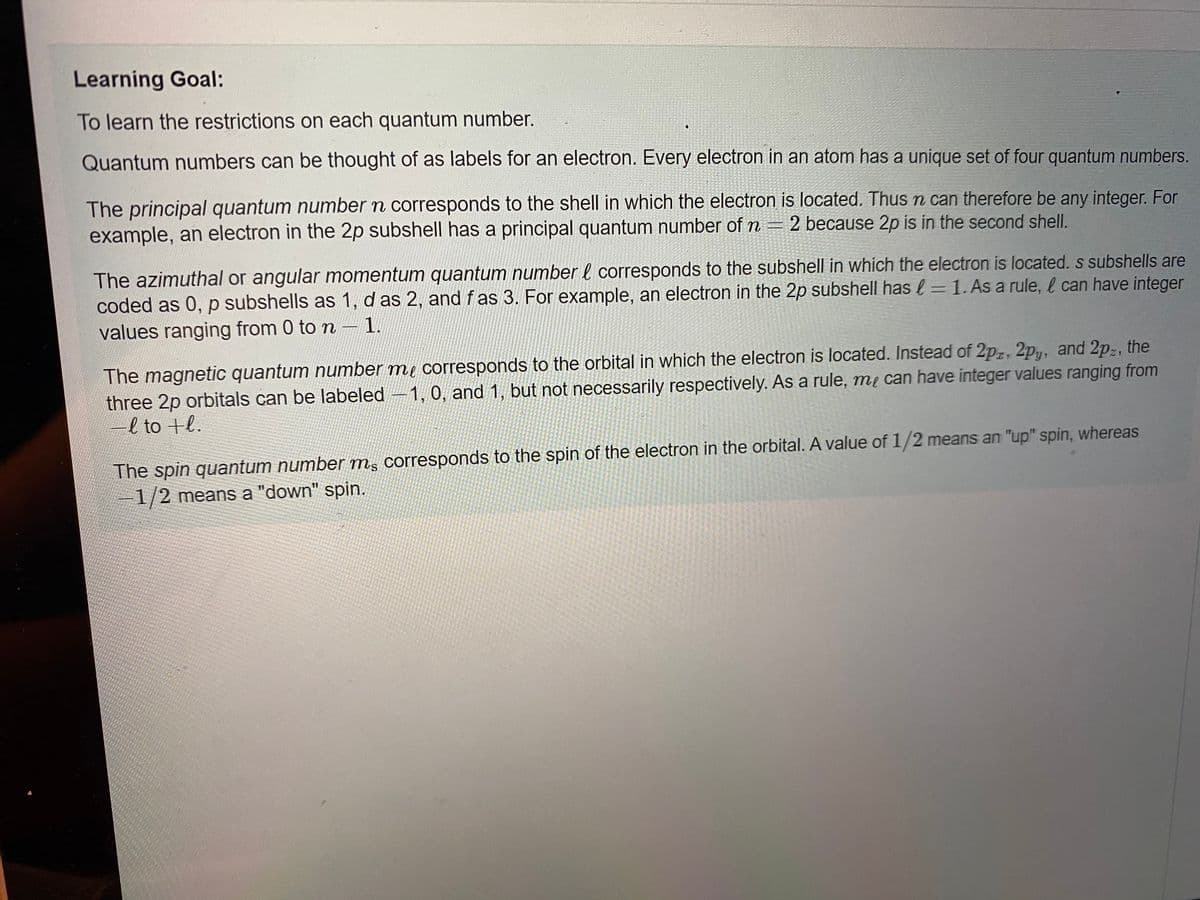
To learn the restrictions on each quantum number.
Quantum numbers can be thought of as labels for an electron. Every electron in an atom has a unique set of four quantum numbers.
The principal quantum number n corresponds to the shell in which the electron is located. Thus n can therefore be any integer. For
example, an electron in the 2p subshell has a principal quantum number of n = 2 because 2p is in the second shell.
The azimuthal or angular momentum quantum number l corresponds to the subshell in which the electron is located. s subshells are
coded as 0, p subshells as 1, d as 2, andf as 3. For example, an electron in the 2p subshell has l = 1. As a rule, l can have integer
values ranging from 0 to n-1.
The magnetic quantum number me corresponds to the orbital in which the electron is located. Instead of 2pz, 2py, and 2p:, the
three 2p orbitals can be labeled -1, 0, and 1, but not necessarily respectively. As a rule, mẹ can have integer values ranging from
-l to +l.
The spin quantum number m, corresponds to the spin of the electron in the orbital. A value of 1/2 means an "up" spin, whereas
1/2 means a "down" spin.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning