PROBLEM: Choose the member with the higher entropy in each of the following pairs, and justify your choice [assume constant temperature, except in part (e)]: (a) 1 mol of SO₂(g) or 1 mol of SO3(g) (b) 1 mol of CO₂(s) or 1 mol of CO₂(g) (c) 3 mol of O₂(g) or 2 mol of O3(g) (d) 1 mol of KBr(s) or 1 mol of KBr(aq) seawater at 2°C or at 23°C (e) (f) 1 mol of CF4(g) or 1 mol of CCl4(9)
PROBLEM: Choose the member with the higher entropy in each of the following pairs, and justify your choice [assume constant temperature, except in part (e)]: (a) 1 mol of SO₂(g) or 1 mol of SO3(g) (b) 1 mol of CO₂(s) or 1 mol of CO₂(g) (c) 3 mol of O₂(g) or 2 mol of O3(g) (d) 1 mol of KBr(s) or 1 mol of KBr(aq) seawater at 2°C or at 23°C (e) (f) 1 mol of CF4(g) or 1 mol of CCl4(9)
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter3: The Second And Third Laws Of Thermodynamics
Section: Chapter Questions
Problem 3.51E: Order the following substances in order of increasing entropy: NaCl(solid), C(graphite), C(diamond),...
Related questions
Question
![PROBLEM: Choose the member with the higher entropy in each of the
following pairs, and justify your choice [assume constant
temperature, except in part (e)]:
(a) 1 mol of SO₂(g) or 1 mol of SO3(g)
(b)
1 mol of CO₂ (s) or 1 mol of CO₂(g)
3 mol of O₂(g) or 2 mol of O3(g)
(c)
(d)
1 mol of KBr(s) or 1 mol of KBr(aq)
seawater at 2°C or at 23°C
(e)
(f) 1 mol of CF₂(g) or 1 mol of CCl4 (9)
PROBLEM: A key step in the production of sulfuric acid is the oxidation
of SO₂(g) to SO3(g):
2SO₂(g) + O₂(g) → 2SO3(g)
At 298 K, AG = -141.6 kJ; AH = -198.4 kJ; and AS = -187.9 J/K
(a) Use the data to decide if this reaction is spontaneous at 25°C, and
predict how AG will change with increasing T.
(b) Assuming AH and AS are constant with increasing T, is the
reaction spontaneous at 900.° C?
PROBLEM:
ΔΗ = 58.1 kJ
At 25°C (298 K), the reduction of copper(1) oxide is
nonspontaneous AG = 8.9 kJ). Calculate the temperature
at which the reaction becomes spontaneous.
AS = 0.165 kJ/K](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fdb82b1bf-9ecb-4650-bc73-bb0c93237287%2Fb9ae4d8d-6e7c-43ac-a289-b52c16be0ccf%2Fumr9rsd_processed.png&w=3840&q=75)
Transcribed Image Text:PROBLEM: Choose the member with the higher entropy in each of the
following pairs, and justify your choice [assume constant
temperature, except in part (e)]:
(a) 1 mol of SO₂(g) or 1 mol of SO3(g)
(b)
1 mol of CO₂ (s) or 1 mol of CO₂(g)
3 mol of O₂(g) or 2 mol of O3(g)
(c)
(d)
1 mol of KBr(s) or 1 mol of KBr(aq)
seawater at 2°C or at 23°C
(e)
(f) 1 mol of CF₂(g) or 1 mol of CCl4 (9)
PROBLEM: A key step in the production of sulfuric acid is the oxidation
of SO₂(g) to SO3(g):
2SO₂(g) + O₂(g) → 2SO3(g)
At 298 K, AG = -141.6 kJ; AH = -198.4 kJ; and AS = -187.9 J/K
(a) Use the data to decide if this reaction is spontaneous at 25°C, and
predict how AG will change with increasing T.
(b) Assuming AH and AS are constant with increasing T, is the
reaction spontaneous at 900.° C?
PROBLEM:
ΔΗ = 58.1 kJ
At 25°C (298 K), the reduction of copper(1) oxide is
nonspontaneous AG = 8.9 kJ). Calculate the temperature
at which the reaction becomes spontaneous.
AS = 0.165 kJ/K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
hello ! im sorry to ask again but i don't understand your hand writing in this part
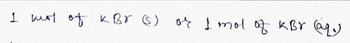
Transcribed Image Text:1 ты от кво () оf f ты оз кву (аду
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co