produces carbon dioxide and water. After 4.64 mL of ethanol (density = 0.789 g/ml) was allowed to burn in the presence of 15.55 g of oxygen gas, 3.70 mL of water (density = 1.00 g/ml) was Part A collected. Determine the limiting reactant for the reaction. (Hint:Write a balanced equation for the combustion of ethanol.) O ethanol O oxygen Part B Determine the theoretical yield of H2O for the reaction. ? m =
produces carbon dioxide and water. After 4.64 mL of ethanol (density = 0.789 g/ml) was allowed to burn in the presence of 15.55 g of oxygen gas, 3.70 mL of water (density = 1.00 g/ml) was Part A collected. Determine the limiting reactant for the reaction. (Hint:Write a balanced equation for the combustion of ethanol.) O ethanol O oxygen Part B Determine the theoretical yield of H2O for the reaction. ? m =
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.76PAE: The pictures below show a molecular-scale view of a chemical reaction between the compounds AB2 and...
Related questions
Question
100%
Please answer question 16 part A, B, and C
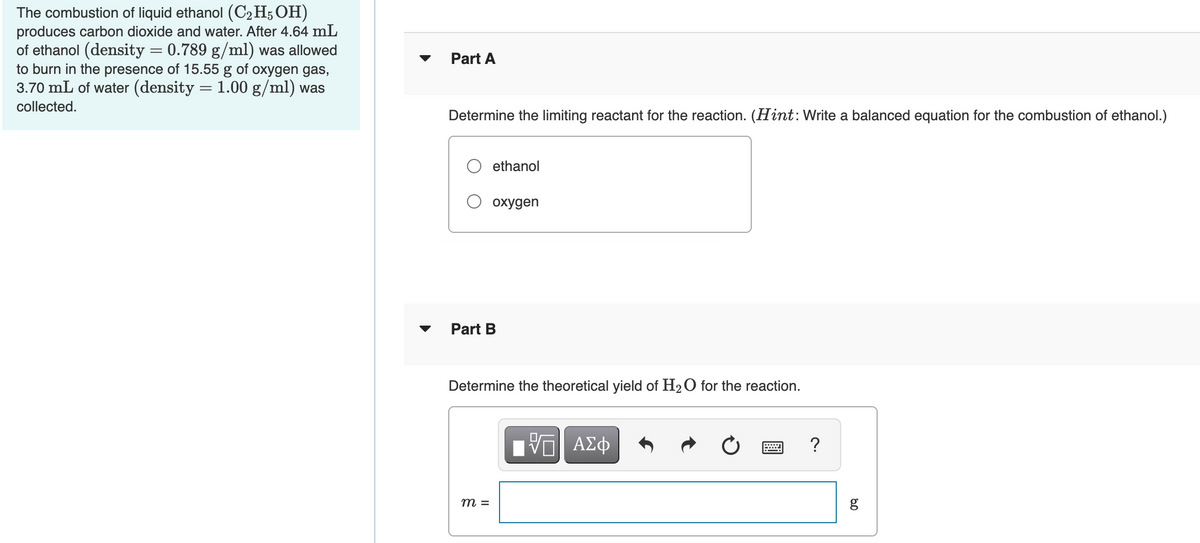
Transcribed Image Text:The combustion of liquid ethanol (C2 H5 OH)
produces carbon dioxide and water. After 4.64 mL
of ethanol (density = 0.789 g/ml) was allowed
to burn in the presence of 15.55 g of oxygen gas,
3.70 mL of water (density
Part A
1.00 g/ml) was
collected.
Determine the limiting reactant for the reaction. (Hint:Write a balanced equation for the combustion of ethanol.)
ethanol
охудen
Part B
Determine the theoretical yield of H20 for the reaction.
?
m =
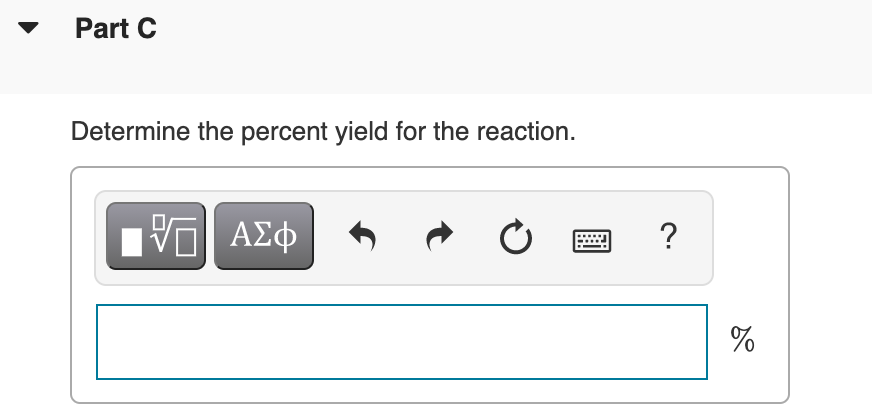
Transcribed Image Text:Part C
Determine the percent yield for the reaction.
?
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
