There are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first step, copper(I) sulfide and oxygen react to form copper(I) oxide and sulfur dioxide: 2Cu,S(s) + 30,(G) 2Cu,0(s) + 2 SO, (G) In the second step, copper(I) oxide and carbon react to form copper and carbon monoxide: Cu,O(s) + C(s) → 2Cu(s) + CO (g) Suppose the yield of the first step is 82.% and the yield of the second step is 83.%. Calculate the mass of oxygen required to make 1.0 kg of copper. Be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits. olo X
There are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first step, copper(I) sulfide and oxygen react to form copper(I) oxide and sulfur dioxide: 2Cu,S(s) + 30,(G) 2Cu,0(s) + 2 SO, (G) In the second step, copper(I) oxide and carbon react to form copper and carbon monoxide: Cu,O(s) + C(s) → 2Cu(s) + CO (g) Suppose the yield of the first step is 82.% and the yield of the second step is 83.%. Calculate the mass of oxygen required to make 1.0 kg of copper. Be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits. olo X
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 7PS: The metals industry was a major source of air pollution years ago. One common process involved...
Related questions
Question
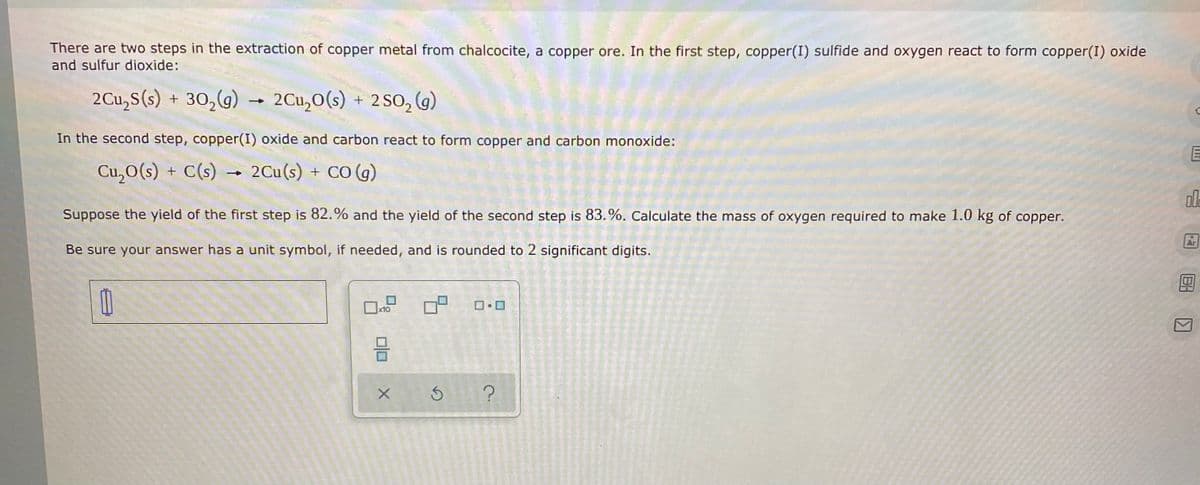
Transcribed Image Text:There are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first step, copper(I) sulfide and oxygen react to form copper(I) oxide
and sulfur dioxide:
2Cu,S(s) + 30,(g) - 2Cu,O(s) + 2SO, (g)
In the second step, copper(I) oxide and carbon react to form copper and carbon monoxide:
Cu,O(s) + C(s) → 2Cu(s) + CO (g)
ol
Suppose the yield of the first step is 82.% and the yield of the second step is 83.%. Calculate the mass of oxygen required to make 1.0 kg of copper.
Be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits.
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning