PROPERTIES OF IONS PRELAB QUESTIONS NAME 1. Define Electrolyte. 2. Solutions that are electrolytes have ions in solution. These ions are surrounded by layers of water molecules known as the solvation sphere. Ions with their solvation spheres are able to move freely about the solution and thus can be capable of transporting an electrical charge. For each of the following compounds identify the ions that will occur in solution. Compound Name Cation(s) Anion(s) 2 Cl(aq) +2 (aq) ВaClz barium chloride Bа Na2S ZnBr2 FeCl3 LIOH AGC2H3O2 Mn(CIO4)2 K,РOд CuCl2 Al(NO3)s Co2(SO4)3 81
PROPERTIES OF IONS PRELAB QUESTIONS NAME 1. Define Electrolyte. 2. Solutions that are electrolytes have ions in solution. These ions are surrounded by layers of water molecules known as the solvation sphere. Ions with their solvation spheres are able to move freely about the solution and thus can be capable of transporting an electrical charge. For each of the following compounds identify the ions that will occur in solution. Compound Name Cation(s) Anion(s) 2 Cl(aq) +2 (aq) ВaClz barium chloride Bа Na2S ZnBr2 FeCl3 LIOH AGC2H3O2 Mn(CIO4)2 K,РOд CuCl2 Al(NO3)s Co2(SO4)3 81
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 66QAP: Assuming that circles represent cations and squares represent anions, match the incomplete net ionic...
Related questions
Question
100%
Help
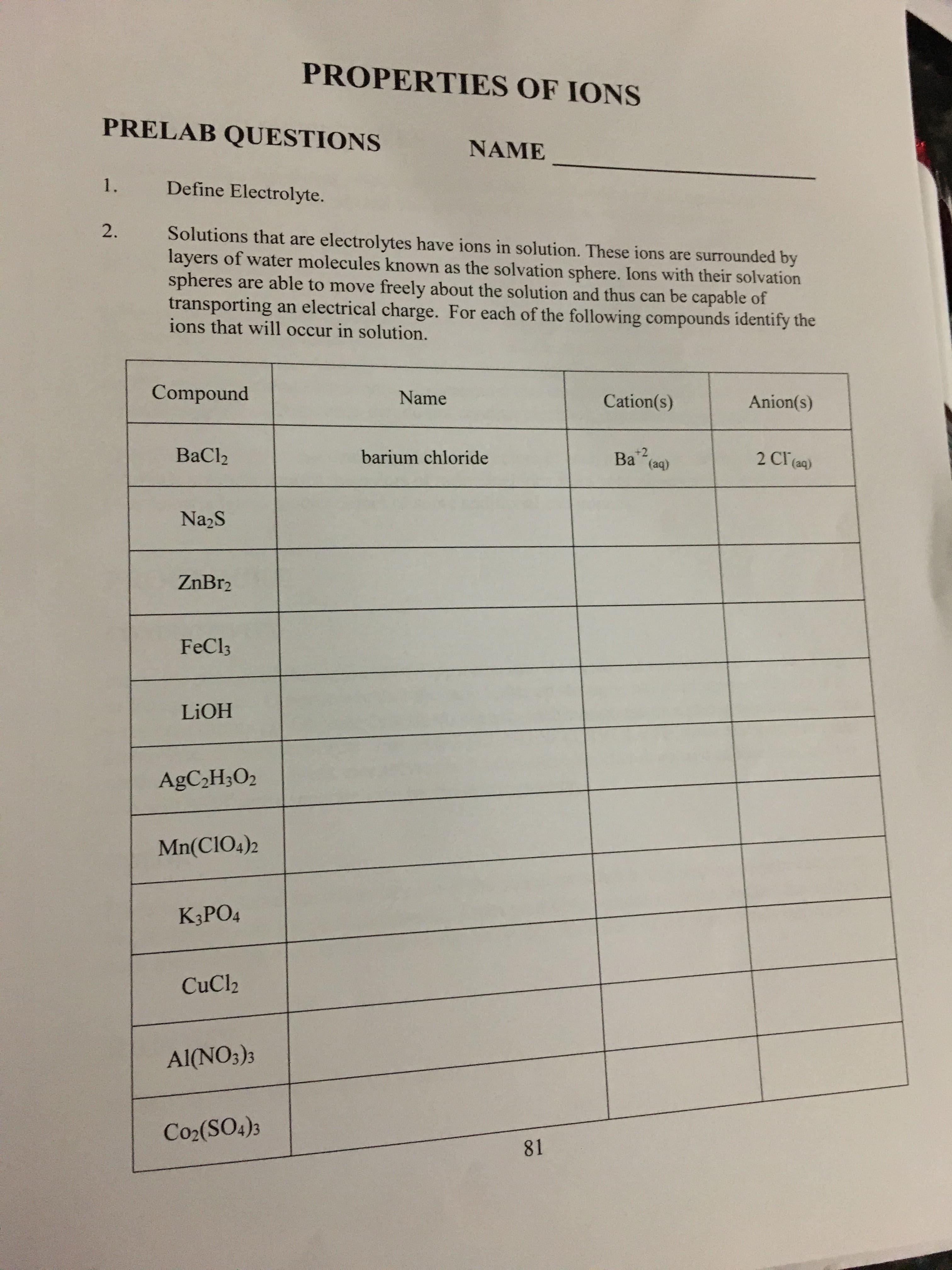
Transcribed Image Text:PROPERTIES OF IONS
PRELAB QUESTIONS
NAME
1.
Define Electrolyte.
2.
Solutions that are electrolytes have ions in solution. These ions are surrounded by
layers of water molecules known as the solvation sphere. Ions with their solvation
spheres are able to move freely about the solution and thus can be capable of
transporting an electrical charge. For each of the following compounds identify the
ions that will occur in solution.
Compound
Name
Cation(s)
Anion(s)
2 Cl(aq)
+2
(aq)
ВaClz
barium chloride
Bа
Na2S
ZnBr2
FeCl3
LIOH
AGC2H3O2
Mn(CIO4)2
K,РOд
CuCl2
Al(NO3)s
Co2(SO4)3
81
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Step 1
VIEWTrending now
This is a popular solution!
Step by step
Solved in 1 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning