Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Some ionic compounds cation anion empirical formula name of compound K* PO Fe+ NO2 NH, BrO,
Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Some ionic compounds cation anion empirical formula name of compound K* PO Fe+ NO2 NH, BrO,
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 113CP: A compound is being tested for use as a rocket propellant. Analysis shows that it contains 18.54% F,...
Related questions
Question
The first one that I sent you guys gave me wrong answers , for this one they ask for the empirical formula and the name of compound please provide the right answers
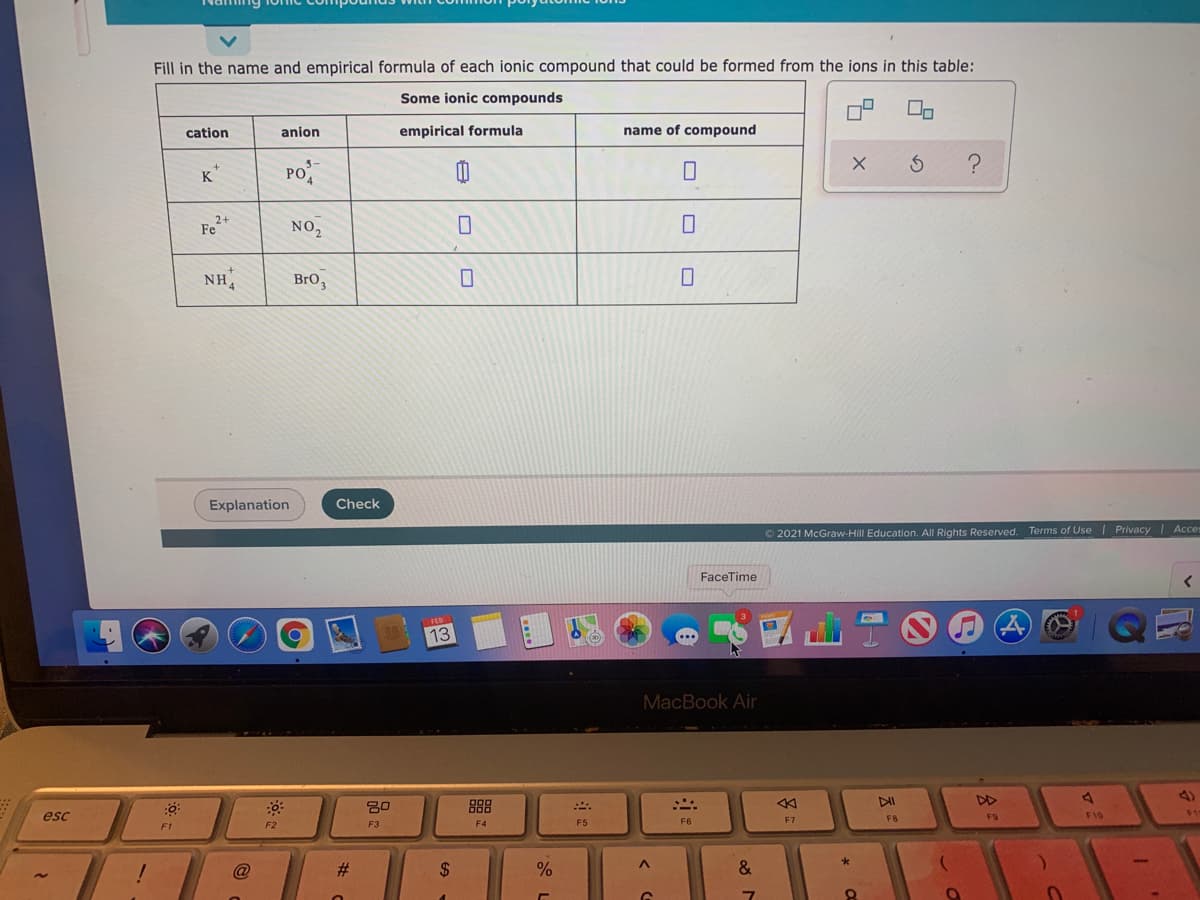
Transcribed Image Text:Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table:
Some ionic compounds
cation
anion
empirical formula
name of compound
K*
Po
Fe
2+
NO,
NH,
BrO3
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Acces
FaceTime
13
MacBook Air
DII
DD
4)
B88
1
esc
F7
FB
F9
F10
F2
F3
F4
F5
F6
F1
%23
%
&
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax