Protein Denatured a. Show that the fraction of denatured macromolecules, 0, is related to the equilibrium constant, Kd, by 1+ K. b. At pH=2, the standard enthalpy and entropy of formation for the denaturation of the enzyme chymotrypsin are 418 kJ/mol and 1.32 kJ K-l mol', respectively. Use these data to generate a plot that shows the temperature dependence of 0. Assume the enthalpy and entropy change to be independent of temperature. c. The "melting temperature" of a biological molecule is defined as the temperature at which 0=1/2. Determine the "melting temperature" of chymotrypsin at pH=2.
Protein Denatured a. Show that the fraction of denatured macromolecules, 0, is related to the equilibrium constant, Kd, by 1+ K. b. At pH=2, the standard enthalpy and entropy of formation for the denaturation of the enzyme chymotrypsin are 418 kJ/mol and 1.32 kJ K-l mol', respectively. Use these data to generate a plot that shows the temperature dependence of 0. Assume the enthalpy and entropy change to be independent of temperature. c. The "melting temperature" of a biological molecule is defined as the temperature at which 0=1/2. Determine the "melting temperature" of chymotrypsin at pH=2.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter16: Spontaneity Of Reaction
Section: Chapter Questions
Problem 78QAP
Related questions
Question
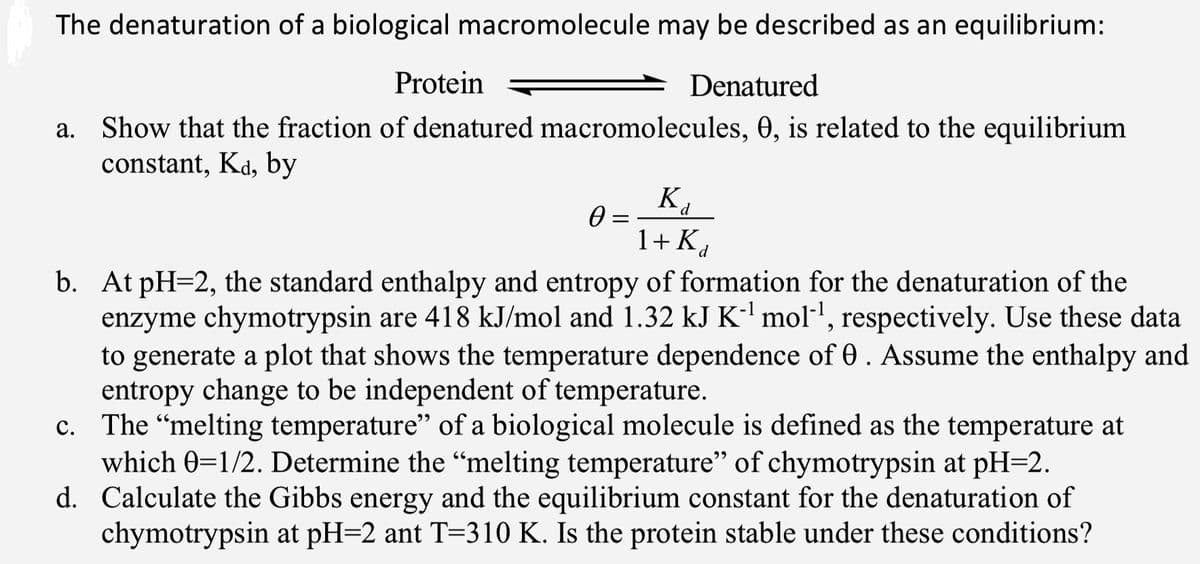
Transcribed Image Text:The denaturation of a biological macromolecule may be described as an equilibrium:
Protein
Denatured
a. Show that the fraction of denatured macromolecules, 0, is related to the equilibrium
constant, Kd, by
K.
1+ Kd
b. At pH=2, the standard enthalpy and entropy of formation for the denaturation of the
enzyme chymotrypsin are 418 kJ/mol and 1.32 kJ K-' mol-', respectively. Use these data
to generate a plot that shows the temperature dependence of 0. Assume the enthalpy and
entropy change to be independent of temperature.
c. The "melting temperature" of a biological molecule is defined as the temperature at
which 0=1/2. Determine the “melting temperature" of chymotrypsin at pH=2.
d. Calculate the Gibbs energy and the equilibrium constant for the denaturation of
chymotrypsin at pH=2 ant T=310 K. Is the protein stable under these conditions?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning