Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section: Chapter Questions
Problem 20.42P: Bicyclo-2,5-heptadiene can be prepared in two steps from cyclopentadiene and vinyl chloride. Provide...
Related questions
Question
Provide Examples of dehydrohalogenation of dihalides to afford
Expert Solution
Step 1
Alkynes-
- Unsaturated hydrocarbon containing carbon carbon triple bond is known as alkyne.
- Alkynes have more electron density so they are highly reactive towards chemical species.
- Alkynes can be easily converted into so many products by using a various chemical reactions in order to obtain the desired products.
- Alkyne consists of ‘sp’ hybridization hence hydrogen attached to triply bonded carbon are most acidic in nature.
- Alkyne is produce from dehydrhalogenation of germinal dihalides and vicinal dihalides.
E2 mechanism- is type of elimination reaction in which two substituents is removed from molecule. It is called bimolecular elimination.
Step 2
Answer of given question:
Alkyne for example 2-butyne is prepared as follows:
When 1, 2 di bromo butane undergo E2 elimination in presence of NaNH2 in excess produce 2-butyne.
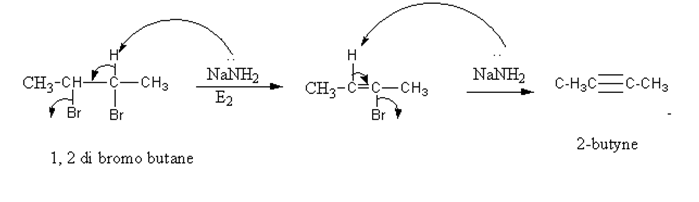
In this reaction from dihaloge, removal of halogen along with hydrogen produce alkyne is known as dehydrohalogenation.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
