Pyridine N-oxides can also react in electrophilic aromatic substitutions. In the reaction below, only a single product is obtained. Use resonance structures to justify the selective formation of this product. NO2 HNO3 H,SO4
Pyridine N-oxides can also react in electrophilic aromatic substitutions. In the reaction below, only a single product is obtained. Use resonance structures to justify the selective formation of this product. NO2 HNO3 H,SO4
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.3: Alkylation And Acylation Of Aromatic Rings: The Friedel–crafts Reaction
Problem 6P: What is the major monosubstitution product from the Friedel—Crafts reaction of benzene with 1...
Related questions
Question
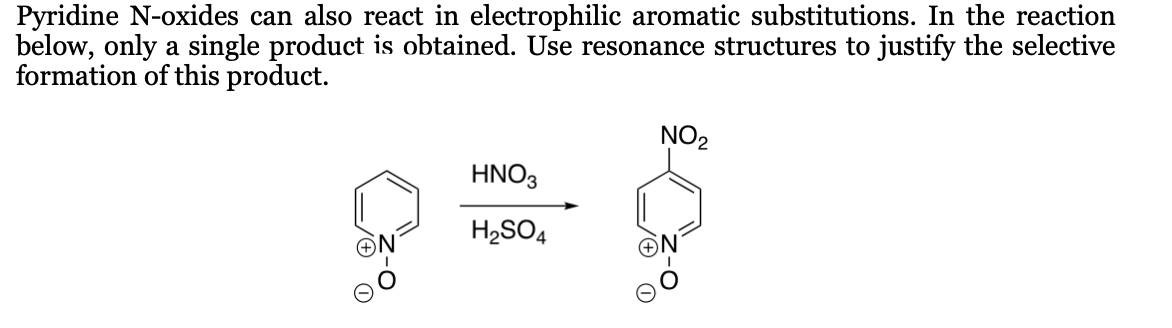
Transcribed Image Text:Pyridine N-oxides can also react in electrophilic aromatic substitutions. In the reaction
below, only a single product is obtained. Use resonance structures to justify the selective
formation of this product.
NO2
HNO3
H2SO4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

