Pyridine undergoes electrophilic aromatic substitution preferentially at the 3 position as illustrated by the synthesis of 3-nitropyridine. NO2 H,SO, + HNO3 + H2O 300°C Pyridine 3-Nitropyridine Under these acidic conditions, the species undergoing nitration is not pyridine, but its conjugate acid. Write resonance contributing structures for the intermediate formed by attack of NO,+ at the 2, 3, and 4 positions of the conjugate acid of pyridine. From examination of these intermediates, offer an explanation for preferential nitration at the 3 position.
Pyridine undergoes electrophilic aromatic substitution preferentially at the 3 position as illustrated by the synthesis of 3-nitropyridine. NO2 H,SO, + HNO3 + H2O 300°C Pyridine 3-Nitropyridine Under these acidic conditions, the species undergoing nitration is not pyridine, but its conjugate acid. Write resonance contributing structures for the intermediate formed by attack of NO,+ at the 2, 3, and 4 positions of the conjugate acid of pyridine. From examination of these intermediates, offer an explanation for preferential nitration at the 3 position.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter22: Reactions Of Benzene And Its Derivatives
Section: Chapter Questions
Problem 22.63P
Related questions
Question
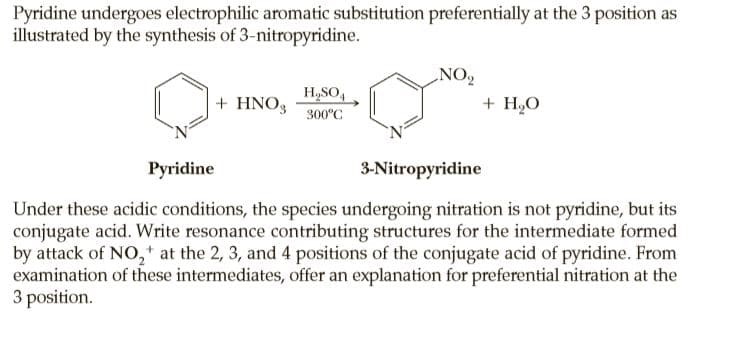
Transcribed Image Text:Pyridine undergoes electrophilic aromatic substitution preferentially at the 3 position as
illustrated by the synthesis of 3-nitropyridine.
NO2
H,SO,
+ HNO3
+ H2O
300°C
Pyridine
3-Nitropyridine
Under these acidic conditions, the species undergoing nitration is not pyridine, but its
conjugate acid. Write resonance contributing structures for the intermediate formed
by attack of NO,+ at the 2, 3, and 4 positions of the conjugate acid of pyridine. From
examination of these intermediates, offer an explanation for preferential nitration at the
3 position.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning