Q: The solubility of hydrogen in water is expressed according to as = 6.8 xlo" Henry's law 6.8x10 X at 20°C, where 015 XH2 is the mole fraction of Ma in the liquid phase and 7s the Partial pressure of Hz (atm) Jf the tolal pressure is 9oo mm Hg, Show that the Henry's law Can be written as Y, = 5.742 xlo XH, where Yu. is the mele fra ction of Hz in the gas phase.
Q: The solubility of hydrogen in water is expressed according to as = 6.8 xlo" Henry's law 6.8x10 X at 20°C, where 015 XH2 is the mole fraction of Ma in the liquid phase and 7s the Partial pressure of Hz (atm) Jf the tolal pressure is 9oo mm Hg, Show that the Henry's law Can be written as Y, = 5.742 xlo XH, where Yu. is the mele fra ction of Hz in the gas phase.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.53QE
Related questions
Question
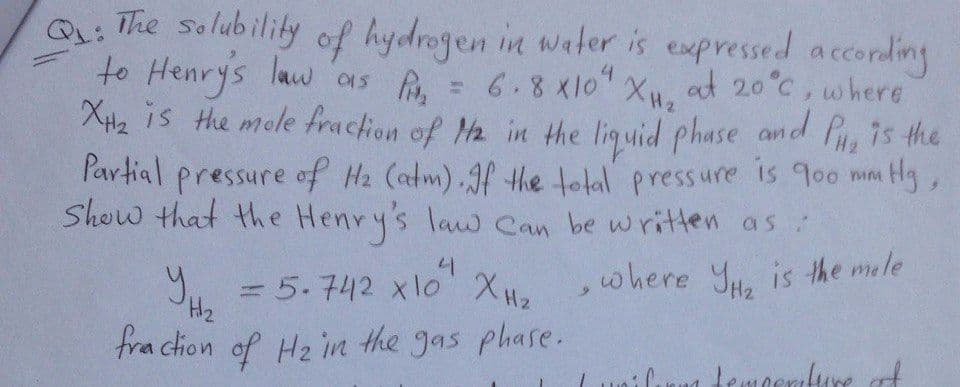
Transcribed Image Text:= 5.742 xlo XH2
Q: The solubility
to Henrys law 01s ,
of hydrogen in water is expressed according
6.8x10 XH2
XH2 is the mole fraction of Ha in the liguid phase and P 7s the
at 20°C, where
P'artial pressure of Hz (atm).Jf the tolal pressure is 900 mm
Show that the Henry's law Can be written as
Hg,
y,
,where Yu. is the mele
H2
fraction of Hz in the gas phase.
ilın temocnure
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning