The %purity of a powdered crude sample of Na2CO3 containing only inert impurities is to be determined by reacting 225.0 mg of the crude sample to 10.0 mL of 3.00 M HCl solution, and bubbling the resulting CO2(g) product in water that is at exactly 29 °C. After the reaction has completed, the level of the liquid inside the eudiometer rests 4.30 cm above the water level in the beaker. The graduation on the eudiometer indicates that the trapped gas is 44.37 mL. The experiment was done under a barometric pressure of 755.2 torr. What CASE does this experiment satisfy?
The %purity of a powdered crude sample of Na2CO3 containing only inert impurities is to be determined by reacting 225.0 mg of the crude sample to 10.0 mL of 3.00 M HCl solution, and bubbling the resulting CO2(g) product in water that is at exactly 29 °C. After the reaction has completed, the level of the liquid inside the eudiometer rests 4.30 cm above the water level in the beaker. The graduation on the eudiometer indicates that the trapped gas is 44.37 mL. The experiment was done under a barometric pressure of 755.2 torr. What CASE does this experiment satisfy?
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter31: Immunochemistry
Section: Chapter Questions
Problem 31.72P
Related questions
Question
The %purity of a powdered crude sample of Na2CO3 containing only inert impurities is to be determined by reacting 225.0 mg of the crude sample to 10.0 mL of 3.00 M HCl solution, and bubbling the resulting CO2(g) product in water that is at exactly 29 °C. After the reaction has completed, the level of the liquid inside the eudiometer rests 4.30 cm above the water level in the beaker. The graduation on the eudiometer indicates that the trapped gas is 44.37 mL. The experiment was done under a barometric pressure of 755.2 torr.
What CASE does this experiment satisfy?
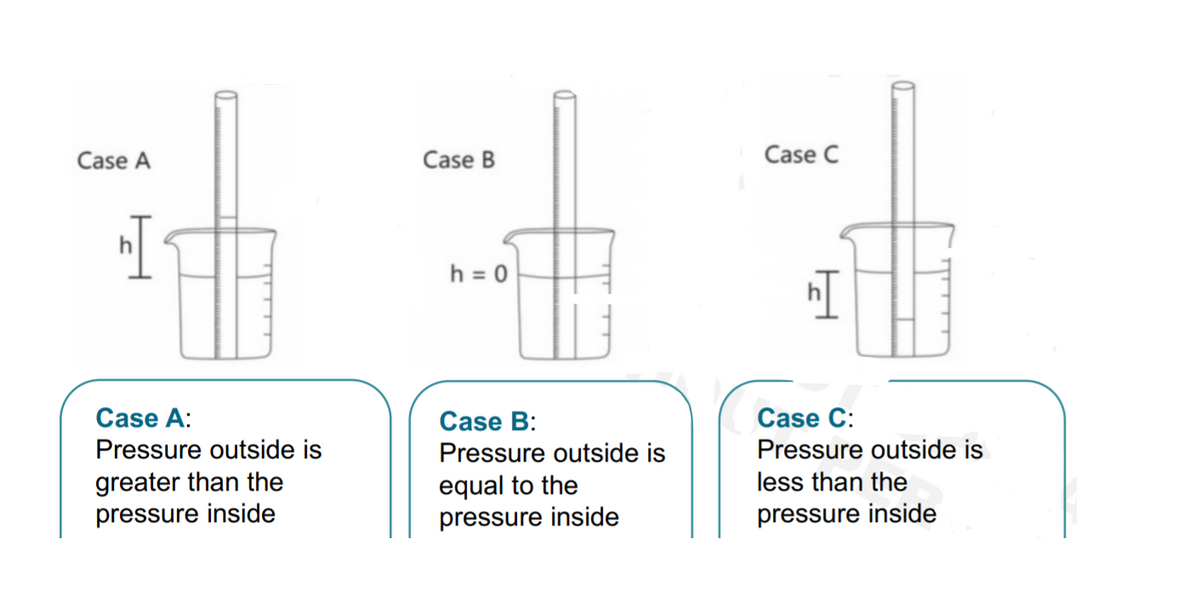
Transcribed Image Text:Case A
Case B
Case C
h = 0
Case A:
Case B:
Case C:
Pressure outside is
Pressure outside is
Pressure outside is
greater than the
equal to the
less than the
pressure inside
pressure inside
pressure inside
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning