Q1)) What do you infer from the figure on the left? 1) A violation of Hund's rule 2) a violation of Pauli's principle of exception. 3) Violation of the FPAO rule. 4) Correct power level chart 2p 2s 1s
Q1)) What do you infer from the figure on the left? 1) A violation of Hund's rule 2) a violation of Pauli's principle of exception. 3) Violation of the FPAO rule. 4) Correct power level chart 2p 2s 1s
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter11: Quantum Mechanics: Model Systems And The Hydrogen Atom
Section: Chapter Questions
Problem 11.92E
Related questions
Question
W1,2,3,4 plz
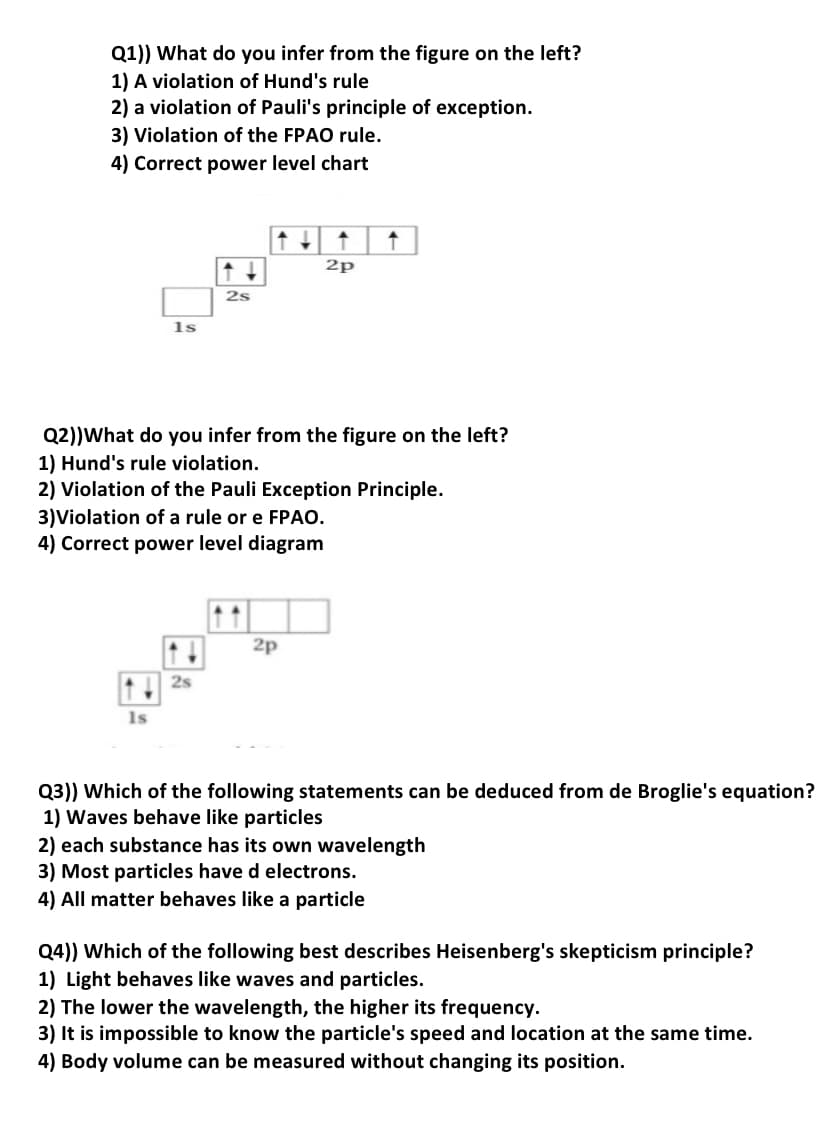
Transcribed Image Text:Q1)) What do you infer from the figure on the left?
1) A violation of Hund's rule
2) a violation of Pauli's principle of exception.
3) Violation of the FPAO rule.
4) Correct power level chart
2p
2s
1s
Q2))What do you infer from the figure on the left?
1) Hund's rule violation.
2) Violation of the Pauli Exception Principle.
3)Violation of a rule or e FPAO.
4) Correct power level diagram
2p
2s
1s
Q3)) Which of the following statements can be deduced from de Broglie's equation?
1) Waves behave like particles
2) each substance has its own wavelength
3) Most particles have d electrons.
4) All matter behaves like a particle
Q4)) Which of the following best describes Heisenberg's skepticism principle?
1) Light behaves like waves and particles.
2) The lower the wavelength, the higher its frequency.
3) It is impossible to know the particle's speed and location at the same time.
4) Body volume can be measured without changing its position.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning