Q11. An automobile travels 97.2 km on 7.88 L of gasoline. What is the gas mileage for the automobile in miles per gallon? (a) 2.02 mi/gal (b) 7.67 mi/gal 20 (c) 0.034 mi/gal(d) 29.0 mi/galm 3
Q11. An automobile travels 97.2 km on 7.88 L of gasoline. What is the gas mileage for the automobile in miles per gallon? (a) 2.02 mi/gal (b) 7.67 mi/gal 20 (c) 0.034 mi/gal(d) 29.0 mi/galm 3
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 38QAP: A gasoline station in Manila, Philippines, charges 38.46 pesos per liter of unleaded gasoline at a...
Related questions
Question
100%
For Q11. I am not getting the correct answer, I am not sure what I’m supposed to do after I convert each unit to the desired unit.
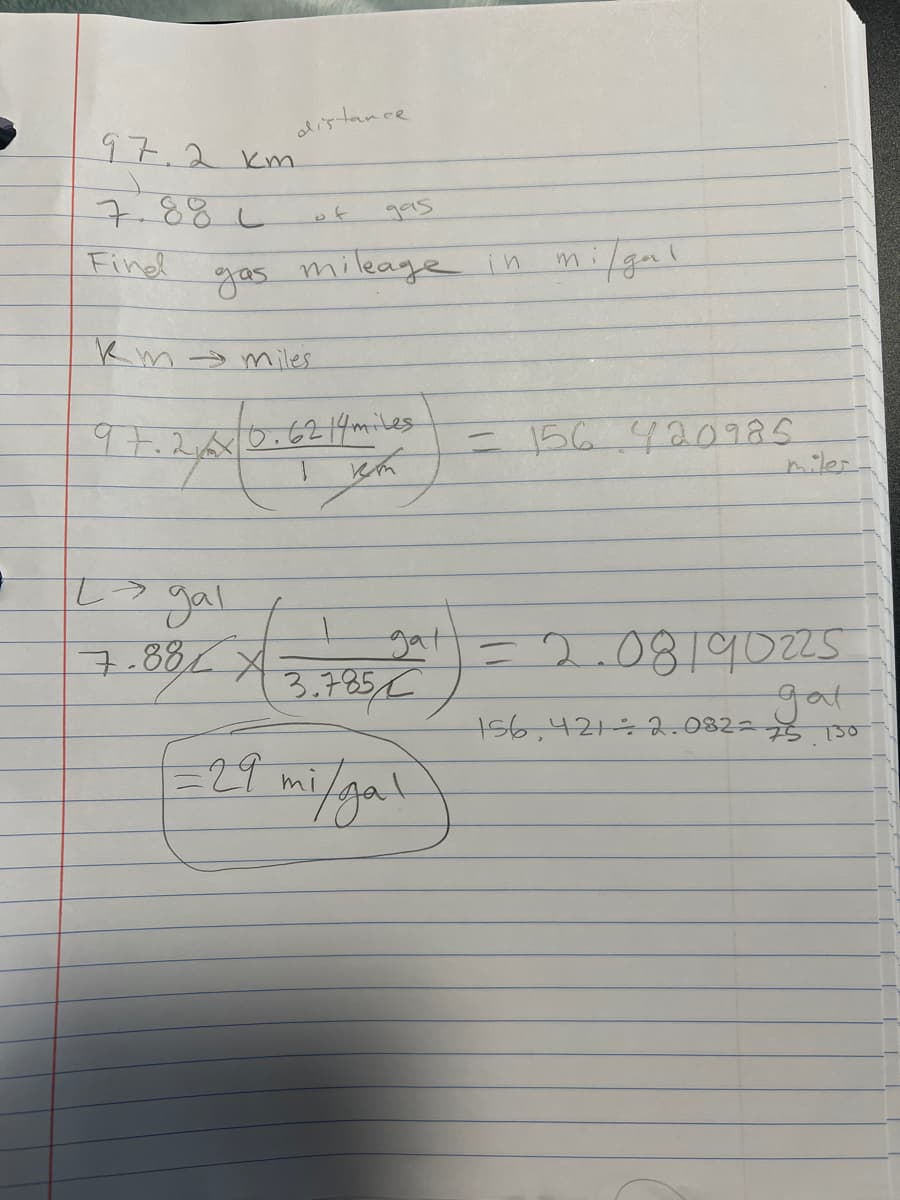
Transcribed Image Text:distance.
995
mileage in mi/gal
97.2 км
7.881
Find
gas
Km → miles
97.2x
0.6214 miles
I кт
L> gal
7.881 3.7851
*
gat
√29 mi/gal
= 156.420985
miles.
= 2.08190225
gat
156,421 ÷ 2.082=75 130

Transcribed Image Text:stering Chemistry™ provides end-of-chapter exercises, feedback-
hed tutorial problems, animations, and interactive activities to encourage
lem solving practice and deeper understanding of key concepts and topics.
au et
on indicates that this feature
interactive in the eText.
amig
Q10. A runner runs 4875 ft in 6.85 minutes. What is the
runner's average speed in miles per hour?
(a) 1.34 mi/hr
(c) 8.09 mi/hr
(b) 0.0022 mi/hr
(d) 8.087 mi/hr
Q11. An automobile travels 97.2 km on 7.88 L of gasoline.
men
What is the gas mileage for the automobile in miles
Eneby
per gallon?
(a) 2.02 mi/gal
(c) 0.034 mi/gal
(b) 7.67 mi/gal
(d) 29.0 mi/gal
Q12. Convert 876.9 in.³ to m³.
(a) 0.01437 m³
(b) 22.27 m³
(d) 0.014 m³
3
(c) 5.351 x 107 m³
Q13. Convert 27 m/s to km/hr.
(a) 97 km/hr
(b) 7.5 km/hr
(c) 1.6 km/hr
(d) 0.027 km/hr
Q14. A cube measures 2.5 cm on each edge and has a mass
of 66.9 g. Calculate the density of the material that com
poses the cube. (The volume of a cube is equal to the
edge length cubed.)
(a) 10.7 g/cm³
(c) 0.234 g/cm³
(b) 4.3 g/cm³
(d) 26.7 g/cm³
Q15. What is the mass of 225 mL of a liquid that has a dens
of 0.880 g/mL?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning