Q2/ Clorine is produced by burning hydrogen cloride gas using air. The reaction taking place in the burner is : 4 HCI (g) + 02 (g) → 2 Cl2 (g) + 2 H,0 (g) Air is used in 35% excess of that theoretically/(stoichiometrically) required. Assuming oxidation to be 80% complete and the dry air and hydrogen chloride gas enter the burner at 298 K (25°C), calculate the adiabatic reaction temperature of the product gas stream. Data : Component AH , kJ/mol H,0 (g) HCI (g) - 241.82 - 92.31 Heat capacity data : C a+bT + cT? + dT³, kJ/(kmol-K) %3D Component a bx 103 сх106 dx 10° HCI 30.3088 - 7.609 13.2608 - 4.3336 - 2.3426 O2 Cl, 26.0257 11.7551 - 0.5623 28.5463 23.8795 - 21.3631 6.4726 - 4.5474 - 4.968 H,O 32.4921 0.0796 13.2107 N2 29.5909 - 5.141 13.1829
Q2/ Clorine is produced by burning hydrogen cloride gas using air. The reaction taking place in the burner is : 4 HCI (g) + 02 (g) → 2 Cl2 (g) + 2 H,0 (g) Air is used in 35% excess of that theoretically/(stoichiometrically) required. Assuming oxidation to be 80% complete and the dry air and hydrogen chloride gas enter the burner at 298 K (25°C), calculate the adiabatic reaction temperature of the product gas stream. Data : Component AH , kJ/mol H,0 (g) HCI (g) - 241.82 - 92.31 Heat capacity data : C a+bT + cT? + dT³, kJ/(kmol-K) %3D Component a bx 103 сх106 dx 10° HCI 30.3088 - 7.609 13.2608 - 4.3336 - 2.3426 O2 Cl, 26.0257 11.7551 - 0.5623 28.5463 23.8795 - 21.3631 6.4726 - 4.5474 - 4.968 H,O 32.4921 0.0796 13.2107 N2 29.5909 - 5.141 13.1829
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section: Chapter Questions
Problem 58QRT: Hydrazine, N2H4, has been proposed as the fuel in a fuel cell in which oxygen is the oxidizing...
Related questions
Question
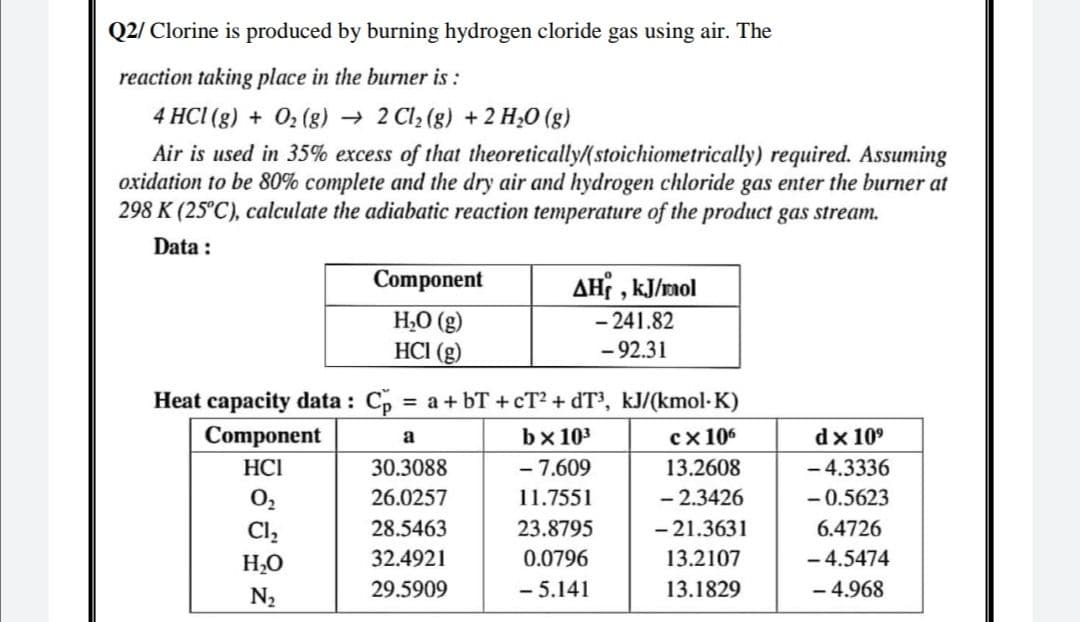
Transcribed Image Text:Q2/ Clorine is produced by burning hydrogen cloride gas using air. The
reaction taking place in the burner is :
4 HCI (g) + 02 (g) → 2 Cl, (g) + 2 H;0 (g)
Air is used in 35% excess of that theoretically/(stoichiometrically) required. Assuming
oxidation to be 80% complete and the dry air and hydrogen chloride gas enter the burner at
298 K (25°C), calculate the adiabatic reaction temperature of the product gas stream.
Data :
Component
AH , kJ/rmol
H,O (g)
HCI (g)
- 241.82
-92.31
Heat capacity data : Cp = a + bT + cT2 + dT³, kJ/(kmol-K)
Component
bx 103
сх106
dx 10°
a
HCI
30.3088
- 7.609
13.2608
- 4.3336
O2
26.0257
11.7551
- 2.3426
- 0.5623
Cl,
28.5463
23.8795
- 21.3631
6.4726
- 4.5474
- 4.968
H,O
32.4921
0.0796
13.2107
N2
29.5909
-5.141
13.1829
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning