Q2. a) What is Atom Economy? Calculate Atom Economy for the following reactions, Synthesis of 1-bromobutane CH3CH2CH2CH2OH+ NaBr + H,SO4 → CH3CH2CH2CH2B1 + NaHSO4 i) ii) carbon monoxide + water ===> hydrogen + carbon dioxide Preparation of hydrogen
Q2. a) What is Atom Economy? Calculate Atom Economy for the following reactions, Synthesis of 1-bromobutane CH3CH2CH2CH2OH+ NaBr + H,SO4 → CH3CH2CH2CH2B1 + NaHSO4 i) ii) carbon monoxide + water ===> hydrogen + carbon dioxide Preparation of hydrogen
Chapter11: Solving Equilibrium Problems For Complex Systems
Section: Chapter Questions
Problem 11.3QAP
Related questions
Question
Please Answer ASAP within 10 minutes with proper explanation in handwritten form. Don't Answer after 9:55 PM otherwise I will give negative ratings!!
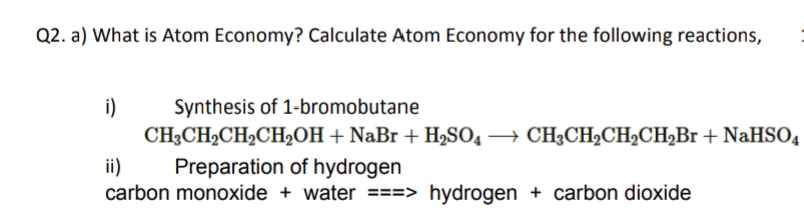
Transcribed Image Text:Q2. a) What is Atom Economy? Calculate Atom Economy for the following reactions,
i)
Synthesis of 1-bromobutane
CH3CH2CH2CH2OH+ NaBr + H2SO4 → CH3CH2CH2CH,Br + NaHSO4
ii)
carbon monoxide + water ===> hydrogen + carbon dioxide
Preparation of hydrogen
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning