Q3. 2.027 g of Salicylic acid was mixed with excess acetic anhydride. (Show ALL steps and mathematical equations involved in calculations. Remember to label all steps clearly and use all appropriate units). a. Calculate the moles of Salicylic acid (provide all answers to 4 decimal places) (2) b. Calculate the theoretical yield of Acetylsalicylic acid (aspirin) in moles (provide all answers to 4 decimal places) (2) c. Calculate the theoretical yield of Acetylsalicylic acid (aspirin) in grams (provide all answers to 3 decimal places) (3) Given information: Salicylic acid: Molar mass of salicylic acid: C7H6O3 (7 x 12.01) + (6 x 1) + (3 x 15.99) = 24.07 + 6 + 47.97 = 132.04 g/mol Acetylsalicylic acid: Molar mass of acetylsalicylic acid: C9H8O4 (9 x 12.01) + (8 x 1) + (4 x 15.99) = 102.09 + 8 + 63.96 = 120.05 g/mol
Q3. 2.027 g of Salicylic acid was mixed with excess acetic anhydride. (Show ALL steps and mathematical equations involved in calculations. Remember to label all steps clearly and use all appropriate units).
a. Calculate the moles of Salicylic acid (provide all answers to 4 decimal places) (2)
b. Calculate the theoretical yield of Acetylsalicylic acid (aspirin) in moles (provide all answers to 4 decimal places) (2)
c. Calculate the theoretical yield of Acetylsalicylic acid (aspirin) in grams (provide all answers to 3 decimal places) (3)
Given information:
Salicylic acid:
Molar mass of salicylic acid: C7H6O3
(7 x 12.01) + (6 x 1) + (3 x 15.99)
= 24.07 + 6 + 47.97
= 132.04 g/mol
Acetylsalicylic acid:
Molar mass of acetylsalicylic acid: C9H8O4
(9 x 12.01) + (8 x 1) + (4 x 15.99)
= 102.09 + 8 + 63.96
= 120.05 g/mol
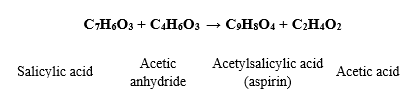
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images









