Q4. In underground coal combustion in the gas phase several reactions ta place including: Co + ½02 → CO2 H2 + ½02 → H20 CH, + 202 → CO2 + 2H20 If a gas phase composed of the following molar composition: CO2: 15.22%; C 13.54%; H2: 15.01%; CHa: 3.2%; and the balance N2, the gas is burned with 40 excess air. On the basis of 100 mol of aas calculate the amount of gir need
Q4. In underground coal combustion in the gas phase several reactions ta place including: Co + ½02 → CO2 H2 + ½02 → H20 CH, + 202 → CO2 + 2H20 If a gas phase composed of the following molar composition: CO2: 15.22%; C 13.54%; H2: 15.01%; CHa: 3.2%; and the balance N2, the gas is burned with 40 excess air. On the basis of 100 mol of aas calculate the amount of gir need
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section10.2: U. S/ Energy Sources And Consumption
Problem 10.4E
Related questions
Question
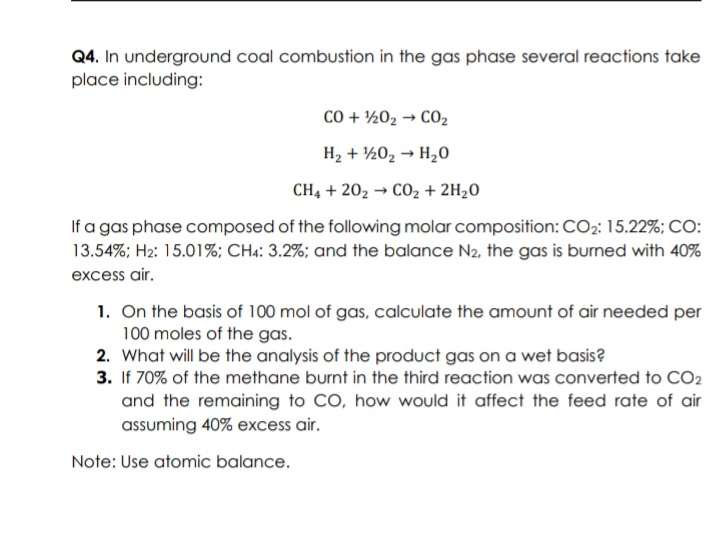
Transcribed Image Text:Q4. In underground coal combustion in the gas phase several reactions take
place including:
CO + ½02 → CO2
H2 + ½02 → H20
CH, + 202 → CO2 + 2H2O
If a gas phase composed of the following molar composition: CO2: 15.22%; CO:
13.54%; H2: 15.01%; CH4: 3.2%; and the balance N2, the gas is burned with 40%
excess air.
1. On the basis of 100 mol of gas, calculate the amount of air needed per
100 moles of the gas.
2. What will be the analysis of the product gas on a wet basis?
3. If 70% of the methane burnt in the third reaction was converted to CO2
and the remaining to CO, how would it affect the feed rate of air
assuming 40% excess air.
Note: Use atomic balance.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning