Where stated, classify reactions both by the key reaction event (PPT, A/B, R/O) and by changes in composition (COM, DEC, SDP, DDP).| 5. Mg(N3)2(s) →– Mg3N2(s) + N2(g) balance, then classify: Calculate the following, with proper number of significant figures: When 0.057 moles of N2 form, moles of Mg3N2 form also. To make 92.0 moles of Mg3N2, moles of Mg(N3)2 need to react. To make 0.48 grams of N2, moles of Mg(N3)2 need to react. When 24.5 grams of Mg(N3)2 react, grams of Mg3N2 are made. To make a count of 5.62x1020 of Mg3N2 units, grams of Mg(N3)2 need to react.
Where stated, classify reactions both by the key reaction event (PPT, A/B, R/O) and by changes in composition (COM, DEC, SDP, DDP).| 5. Mg(N3)2(s) →– Mg3N2(s) + N2(g) balance, then classify: Calculate the following, with proper number of significant figures: When 0.057 moles of N2 form, moles of Mg3N2 form also. To make 92.0 moles of Mg3N2, moles of Mg(N3)2 need to react. To make 0.48 grams of N2, moles of Mg(N3)2 need to react. When 24.5 grams of Mg(N3)2 react, grams of Mg3N2 are made. To make a count of 5.62x1020 of Mg3N2 units, grams of Mg(N3)2 need to react.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 50A
Related questions
Question
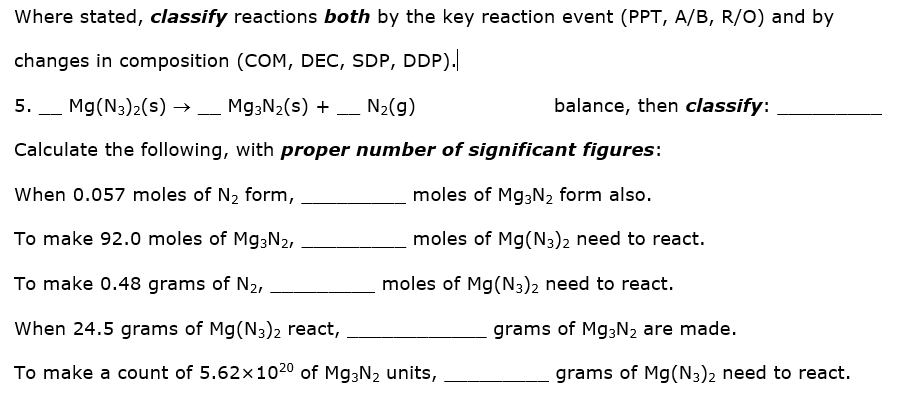
Transcribed Image Text:Where stated, classify reactions both by the key reaction event (PPT, A/B, R/O) and by
changes in composition (COM, DEC, SDP, DDP).
5. _ Mg(N3)2(s) → _ M93N2(s) + _ N2(g)
balance, then classify:
-
Calculate the following, with proper number of significant figures:
When 0.057 moles of N2 form,
moles of Mg;N2 form also.
To make 92.0 moles of Mg3N2,
moles of Mg(N3)2 need to react.
To make 0.48 grams of N2,
moles of Mg(N3)2 need to react.
When 24.5 grams of Mg(N3)2 react,
grams of M93N2 are made.
To make a count of 5.62x1020 of Mg3N2 units,
grams of Mg(N3)2 need to react.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning