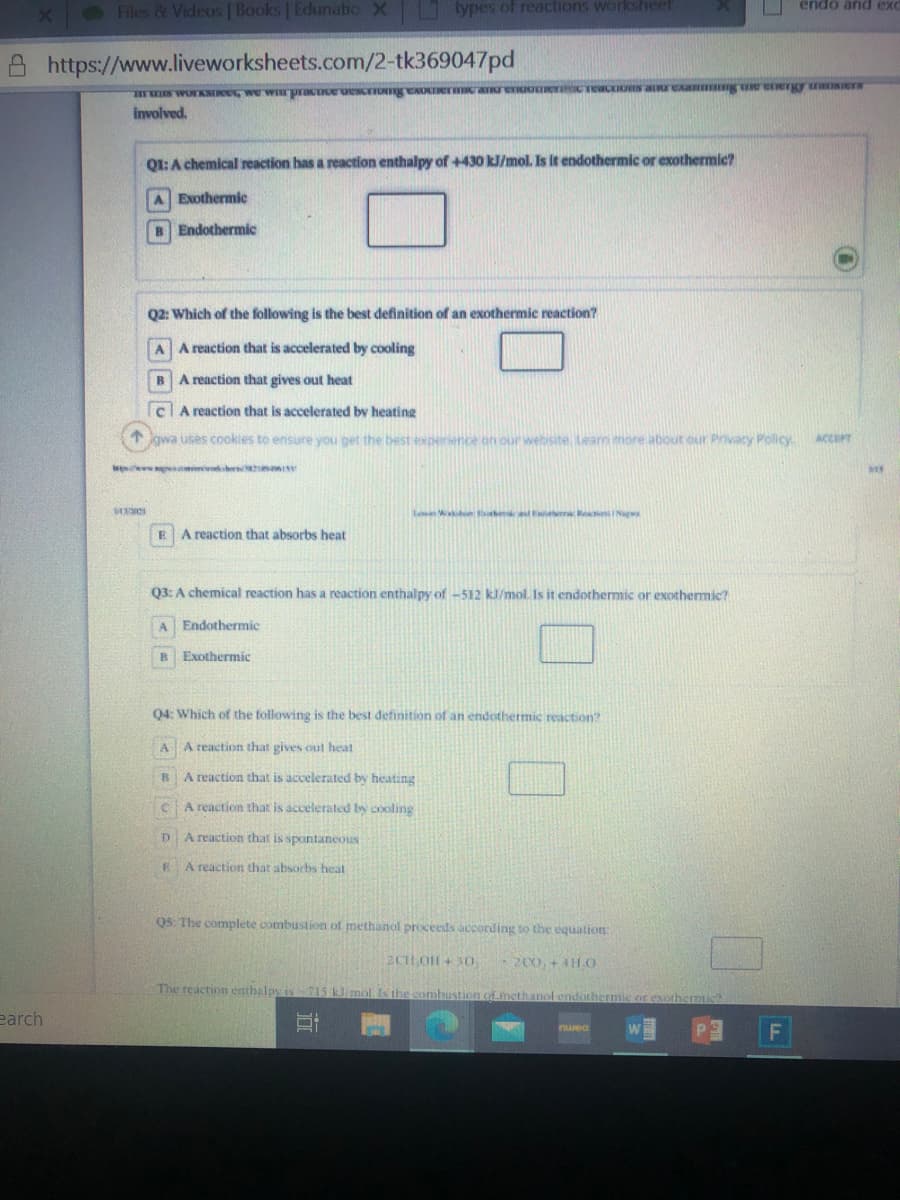
QI: A chemical reaction has a reaction enthalpy of +430 kJ/mol. Is it endothermic or exothermic? Exothermic Endothermic Q2: Which of the following is the best definition of an exothermic reaction? A A reaction that is accelerated by cooling BA reaction that gives out heat A reaction that is accelerated by heating igwa uses cookies to ensure you get the best experience on our webisite earn more about our Privacy Policy ACCEPT e W eR INgs E A reaction that absorbs heat Q3: A chemical reaction has a reaction enthalpy of -512 kl/mol. Is it endothermic or exothermic? A Endothermic B Exothermic
QI: A chemical reaction has a reaction enthalpy of +430 kJ/mol. Is it endothermic or exothermic? Exothermic Endothermic Q2: Which of the following is the best definition of an exothermic reaction? A A reaction that is accelerated by cooling BA reaction that gives out heat A reaction that is accelerated by heating igwa uses cookies to ensure you get the best experience on our webisite earn more about our Privacy Policy ACCEPT e W eR INgs E A reaction that absorbs heat Q3: A chemical reaction has a reaction enthalpy of -512 kl/mol. Is it endothermic or exothermic? A Endothermic B Exothermic
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
SectionU5.12: Over The Hill: Reversing Reactions
Problem 4E
Related questions
Question
Transcribed Image Text:Files & Videos | Books | Edunatio X
types of reactions worksheef
endo and ex
https://www.liveworksheets.com/2-tk369047pd
involved.
QI:A chemical reaction has a reaction enthalpy of +430 kJ/mol. Is it endothermic or exothermic?
A Exothermic
B Endothermic
Q2: Which of the following is the best definition of an exothermic reaction?
A A reaction that is accelerated by cooling
A reaction that gives out heat
clA reaction that is accelerated by heating
gwa uses cookies to ensure you get the best experience
vensite Leam more about our Privacy Policy
ACCEPT
w omimvers
Lean he dee R INag
A reaction that absorbs heat
Q3: A chemical reaction has a reaction enthalpy of -512 kJ/mol. Is it endothermic or exothermic?
Endothermic
Exothermic
Q4: Which of the following is the best definition of an endothermic reaction?
AA reaction that gives out heat
A reaction that is accelerated by heating
C.
A reaction that is accelerated by cooling
D.
A reaction that is spontaneous
A reaction that absorbs heat
Q5: The complete combustion of methanol proceeds according to the equation:
2CH OH+ 3O.
-2C0. + 4HHO
The reaction enthalpy is 715 k molIs the combustion ofmethanol endothermic or exothermic
earch
F
Damu
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning