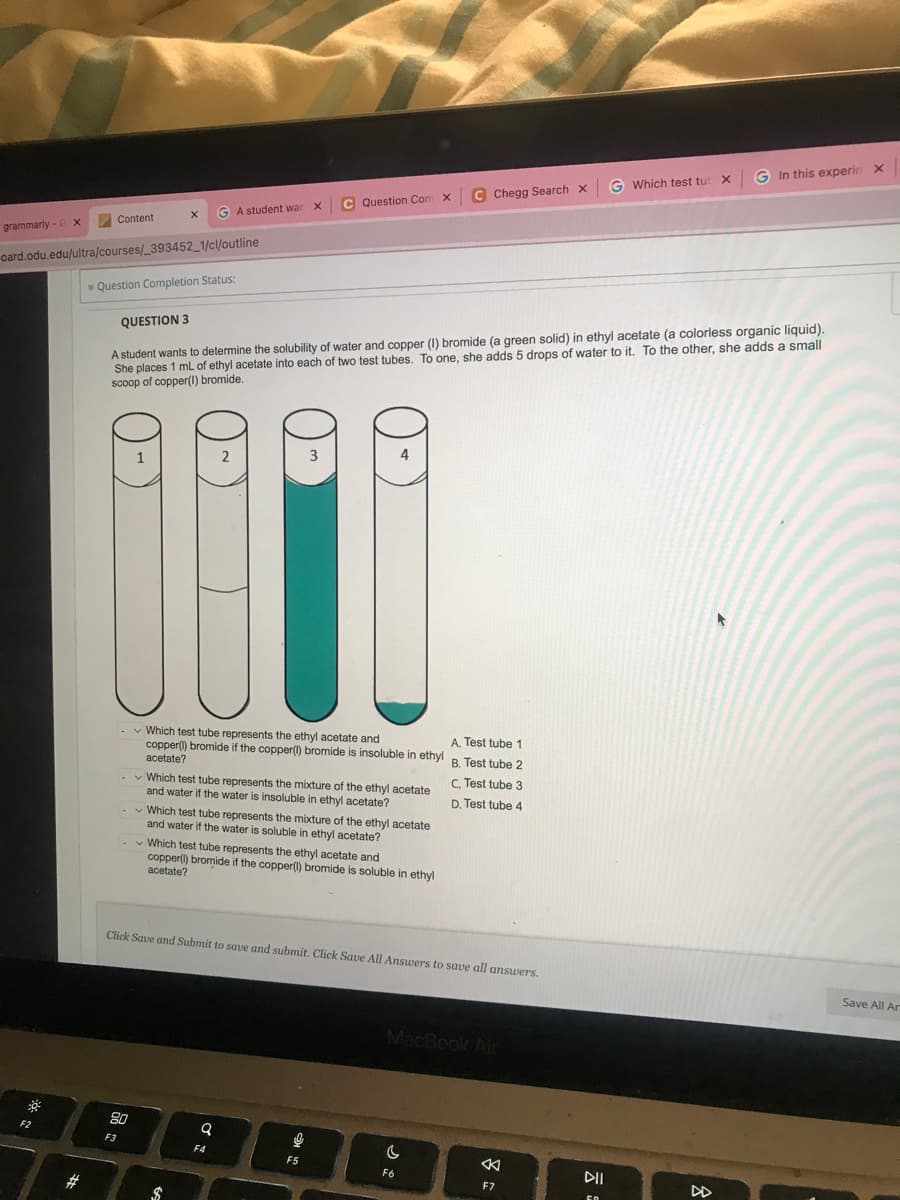
Que QUESTION 3 A student wants to determine the solubility of water and copper (1) bromide (a green solid) in ethyl acetate (a colorless organic liquid). She places 1 mL of ethyl acetate into each of two test tubes. To one, she adds 5 drops of water to it. To the other, she adds a small scoop of copper(1) bromide. 2 3 Which test tube represents the ethyl acetate and copper(1) bromide if the copper(1) bromide is insoluble in ethyl acetate? Which test tube represents the mixture of the ethyl acetate and water if the water is insoluble in ethyl acetate? Which test tube represents the mixture of the ethyl acetate and water if the water is soluble in ethyl acetate? Which test tube represents the ethyl acetate and copper() bromide if the copper(1) bromide is soluble in ethyl acetate? B. Test tube 2 A. Test tube 1 C. Test tube 3 D. Test tube 4
Que QUESTION 3 A student wants to determine the solubility of water and copper (1) bromide (a green solid) in ethyl acetate (a colorless organic liquid). She places 1 mL of ethyl acetate into each of two test tubes. To one, she adds 5 drops of water to it. To the other, she adds a small scoop of copper(1) bromide. 2 3 Which test tube represents the ethyl acetate and copper(1) bromide if the copper(1) bromide is insoluble in ethyl acetate? Which test tube represents the mixture of the ethyl acetate and water if the water is insoluble in ethyl acetate? Which test tube represents the mixture of the ethyl acetate and water if the water is soluble in ethyl acetate? Which test tube represents the ethyl acetate and copper() bromide if the copper(1) bromide is soluble in ethyl acetate? B. Test tube 2 A. Test tube 1 C. Test tube 3 D. Test tube 4
Chapter79: Solubility
Section: Chapter Questions
Problem 1P
Related questions
Question
Transcribed Image Text:grammarly-G X
*
Content
oard.odu.edu/ultra/courses/_393452_1/cl/outline
F2
Question Completion Status:
X GA student wan X
1
QUESTION 3
A student wants to determine the solubility of water and copper (1) bromide (a green solid) in ethyl acetate (a colorless organic liquid).
She places 1 mL of ethyl acetate into each of two test tubes. To one, she adds 5 drops of water to it. To the other, she adds a small
scoop of copper(1) bromide.
8.0
F3
2
- Which test tube represents the ethyl acetate and
A. Test tube 1
copper(1) bromide if the copper(1) bromide is insoluble in ethyl B. Test tube 2
acetate?
C. Test tube 3
D. Test tube 4
- Which test tube represents the mixture of the ethyl acetate
and water if the water is insoluble in ethyl acetate?
$
Question Com X C Chegg Search x
3
Which test tube represents the mixture of the ethyl acetate
and water if the water is soluble in ethyl acetate?
- Which test tube represents the ethyl acetate and
copper(1) bromide if the copper(1) bromide is soluble in ethyl
acetate?
Q
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
F4
4
9
F5
MacBook Air
C
F6
F7
DII
G Which test tut X
CO
G In this experin X
DD
Save All Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning