Question 1 1 Consider the following reaction: Ce2(g) + 2NO(g) = 2NOC{(g) 1.1 Determine the estimated value of the equilibrium constant for this reaction at 158 °C by making use of the following data: kj at 25°C mol Substance at 25°C 4,H° ()a \mol. K. Cl2(g) 221.3 NO(g) 210.8 90.29 NOC? (g) 261.7 51.71 1.2 Based on your final answer in question 1.1, is the reaction reactant- or product favoured at this temperature? Use a short sentence to explain your answer. 1.3 Calculate the value of A,G for this reaction at this temperature, if Cł2 , NO and NOC? are present at 0.600 atm, 0.700 atm and 0.500 atm, respectively.
Question 1 1 Consider the following reaction: Ce2(g) + 2NO(g) = 2NOC{(g) 1.1 Determine the estimated value of the equilibrium constant for this reaction at 158 °C by making use of the following data: kj at 25°C mol Substance at 25°C 4,H° ()a \mol. K. Cl2(g) 221.3 NO(g) 210.8 90.29 NOC? (g) 261.7 51.71 1.2 Based on your final answer in question 1.1, is the reaction reactant- or product favoured at this temperature? Use a short sentence to explain your answer. 1.3 Calculate the value of A,G for this reaction at this temperature, if Cł2 , NO and NOC? are present at 0.600 atm, 0.700 atm and 0.500 atm, respectively.
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 20QAP
Related questions
Question
100%
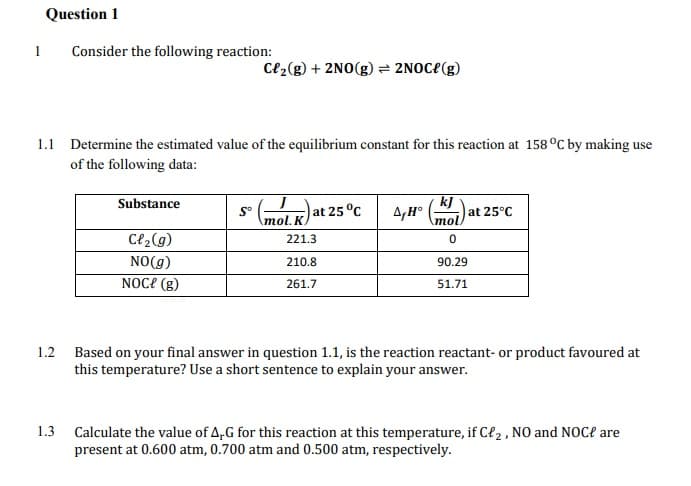
Transcribed Image Text:Question 1
1
Consider the following reaction:
Ce2(g) + 2NO(g) = 2NOC{(g)
1.1 Determine the estimated value of the equilibrium constant for this reaction at 158 °C by making use
of the following data:
kj
at 25°C
mol)
Substance
S°
\mol. K
at 25 °C
C{2(g)
221.3
NO(g)
210.8
90.29
NOCE (g)
261.7
51.71
1.2 Based on your final answer in question 1.1, is the reaction reactant- or product favoured at
this temperature? Use a short sentence to explain your answer.
1.3
Calculate the value of A,G for this reaction at this temperature, if Cł2 , NO and NOC? are
present at 0.600 atm, 0.700 atm and 0.500 atm, respectively.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning