QUESTION 1 A common laboratory reaction is the neutralization of an acid with a base. When 50.0 mL of 0.500 M HCI at 25.0°C is added to 50.0 ml of 0.500 M NaOH at 25.0°C in a coffee cup calorimeter, the temperature of the mixture rises to 28.2°C. What is the heat of reaction per mole of acid? Assume the mixture has a specific heat capacity of 4.18 J/g.°C and that the densities of the reactant solutions are both 1.00 g/mL. O 54 kJ O > 100 kJ O 1300 J 670 J O 27 kJ O O O
QUESTION 1 A common laboratory reaction is the neutralization of an acid with a base. When 50.0 mL of 0.500 M HCI at 25.0°C is added to 50.0 ml of 0.500 M NaOH at 25.0°C in a coffee cup calorimeter, the temperature of the mixture rises to 28.2°C. What is the heat of reaction per mole of acid? Assume the mixture has a specific heat capacity of 4.18 J/g.°C and that the densities of the reactant solutions are both 1.00 g/mL. O 54 kJ O > 100 kJ O 1300 J 670 J O 27 kJ O O O
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 88GQ: You drink 350 mL of diet soda that is at a temperature of 5 C. (a) How much energy will your body...
Related questions
Question
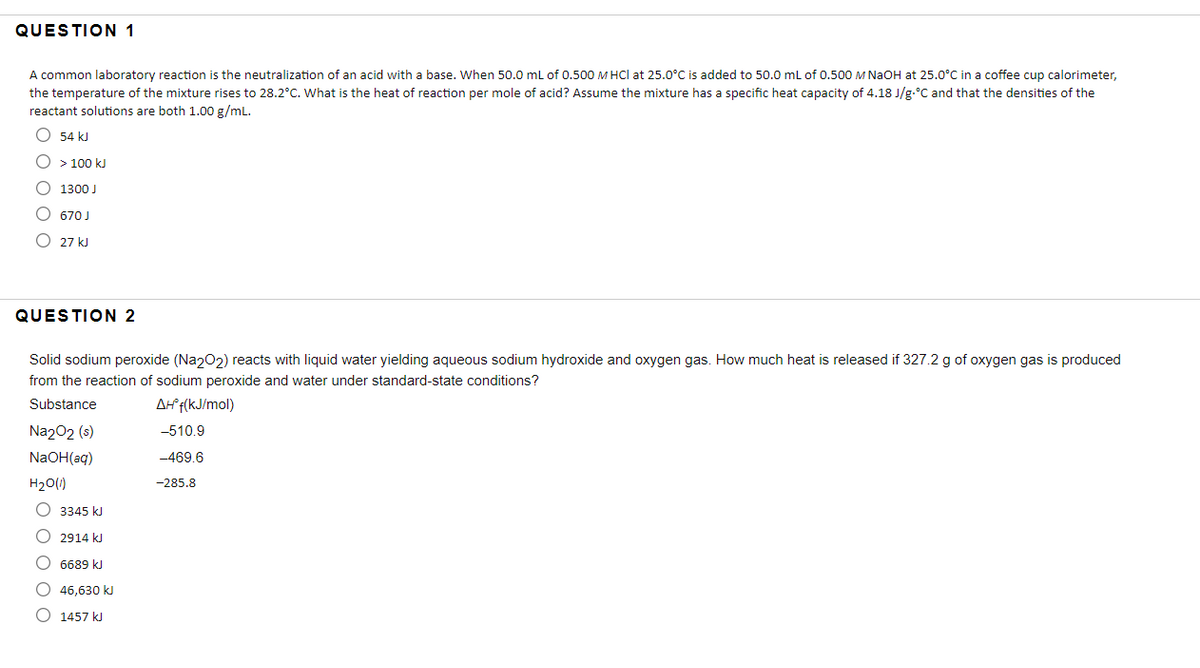
Transcribed Image Text:QUESTION 1
A common laboratory reaction is the neutralization of an acid with a base. When 50.0 mL of 0.500 M HCl at 25.0°C is added to 50.0 mL of 0.500 M NaOH at 25.0°C in a coffee cup calorimeter,
the temperature of the mixture rises to 28.2°C. What is the heat of reaction per mole of acid? Assume the mixture has a specific heat capacity of 4.18 J/g.°C and that the densities of the
reactant solutions are both 1.00 g/mL.
O 54 kJ
O > 100 kJ
O 1300 J
670 J
O 27 kJ
QUESTION 2
Solid sodium peroxide (Na202) reacts with liquid water yielding aqueous sodium hydroxide and oxygen gas. How much heat is released if 327.2 g of oxygen gas is produced
from the reaction of sodium peroxide and water under standard-state conditions?
Substance
Af f(kJ/mol)
Na202 (s)
-510.9
NaOH(aq)
-469.6
H20(1)
-285.8
O 3345 kJ
O 2914 kJ
O 6689 kJ
O 46,630 k
O 1457 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning