Question 1 Read each question carefully. Write your response in the space provided for each part of each question. Answers must be written out in paragraph form. Outlines, bulleted lists, or diagrams alone are not acceptable and will not be scored. The diagram shows water molecules as solid ice at 0"C and as a liquid at 25 C
Question 1 Read each question carefully. Write your response in the space provided for each part of each question. Answers must be written out in paragraph form. Outlines, bulleted lists, or diagrams alone are not acceptable and will not be scored. The diagram shows water molecules as solid ice at 0"C and as a liquid at 25 C
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 56QRT
Related questions
Question
Continuation of the first question part a, b, and c
Transcribed Image Text:1 of 10
Question 1
Read each question carefully. Write your response in the space provided for each part of each question. Answers must be written out in paragraph form. Outlines, bulleted lists, or diagrams alone are not
acceptable and will not be scored.
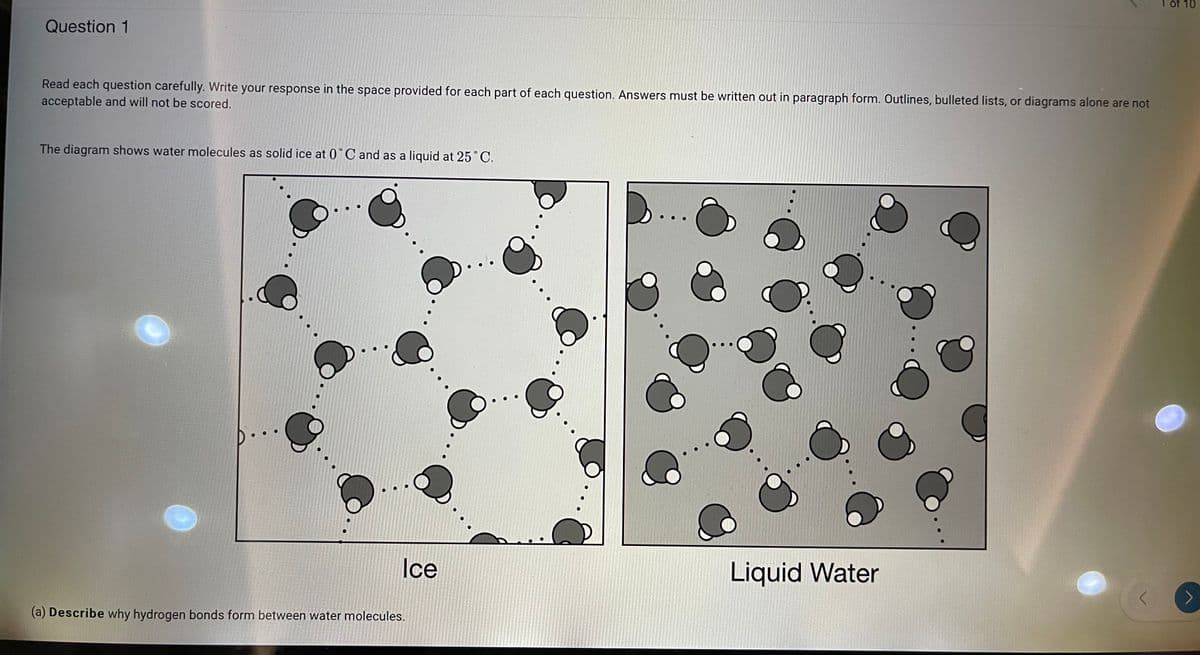
The diagram shows water molecules as solid ice at 0°C and as a liquid at 25°C.
Ice
Liquid Water
(a) Describe why hydrogen bonds form between water molecules.
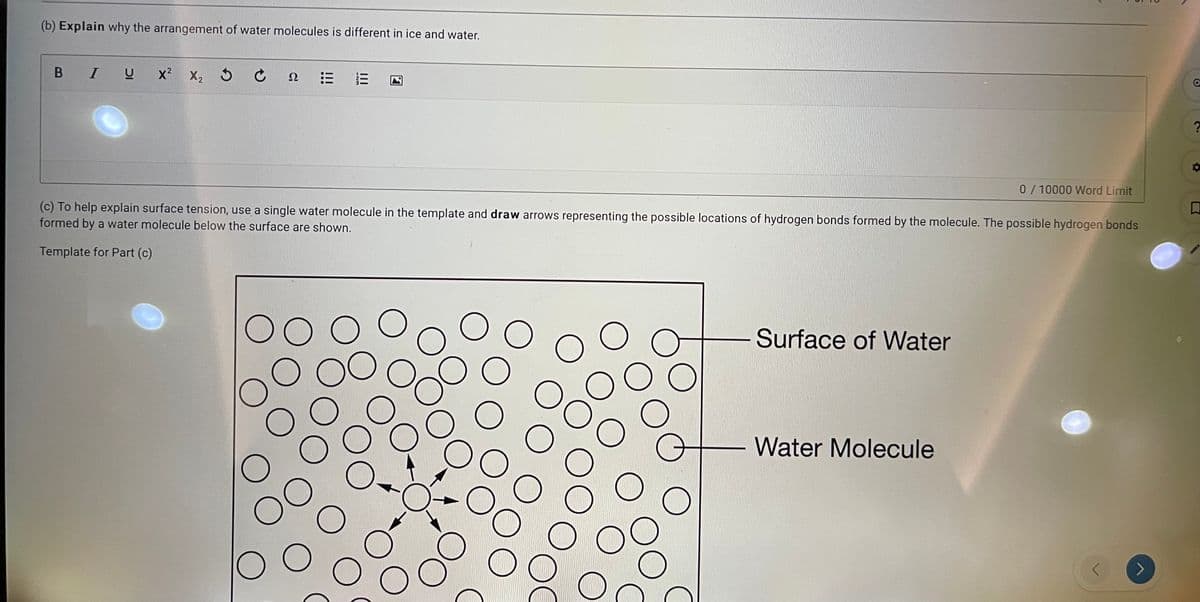
Transcribed Image Text:(b) Explain why the arrangement of water molecules is different in ice and water.
В I U
x? X2 5 C
Ω
0/10000 Word Limit
(c) To help explain surface tension, use a single water molecule in the template and draw arrows representing the possible locations of hydrogen bonds formed by the molecule. The possible hydrogen bonds
formed by a water molecule below the surface are shown.
Template for Part (c)
00
Surface of Water
OC
Water Molecule
!!!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning