1. Draw particulate representations of the three other ionic compounds in the table above, Be sure to represent both the charges of the ions and their sizes in your drawings. Mgo Nal NaBr 2. Use the particulate representations you have drawn and Coulomb's law to explain the trends in boiling point shown in the table above.
1. Draw particulate representations of the three other ionic compounds in the table above, Be sure to represent both the charges of the ions and their sizes in your drawings. Mgo Nal NaBr 2. Use the particulate representations you have drawn and Coulomb's law to explain the trends in boiling point shown in the table above.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 72QRT
Related questions
Question
The Structure of lonic Compounds/ Exit Ticket
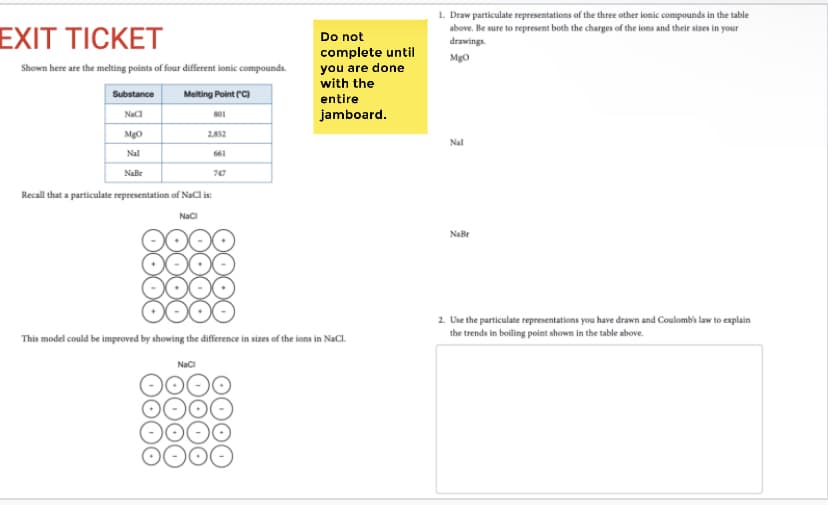
Transcribed Image Text:1. Draw particulate representations of the three other ionic compounds in the table
EXIT TICKET
above. Be sure to represent both the charges of the ions and their sizes in your
drawings.
Do not
complete until
you are done
with the
Mgo
Shown here are the melting points of four different ionic compounds.
Substance
Melting Point rC)
entire
jamboard.
801
Mg0
2.852
Nal
Nal
661
Naße
747
Recall that a particulate representation of NaCl is:
Naci
00
NaBr
2. Use the particulate representations you have drawn and Coulombis law to explain
the trends in boiling point shown in the table above.
This model could be improved by showing the difference in sizes of the ions in NaCl.
Nac
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
