QUESTION 11 Which of the following formula/name pairs are incorrect? More than one may be incorrect. Mark all that are incorect. AN204 dinitrogen tetroxide B. FeCl3 0C. BeO iron trichloride beryllium(II)oxide sulfuric acid cobalt(II) nitrate D.H₂SO4 E. Co(NO3)2 nuation below. Suppose 5.00 g of Li (s) i nur answer in 3 significa
QUESTION 11 Which of the following formula/name pairs are incorrect? More than one may be incorrect. Mark all that are incorect. AN204 dinitrogen tetroxide B. FeCl3 0C. BeO iron trichloride beryllium(II)oxide sulfuric acid cobalt(II) nitrate D.H₂SO4 E. Co(NO3)2 nuation below. Suppose 5.00 g of Li (s) i nur answer in 3 significa
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter5: Nomenclature
Section: Chapter Questions
Problem 27CR
Related questions
Question
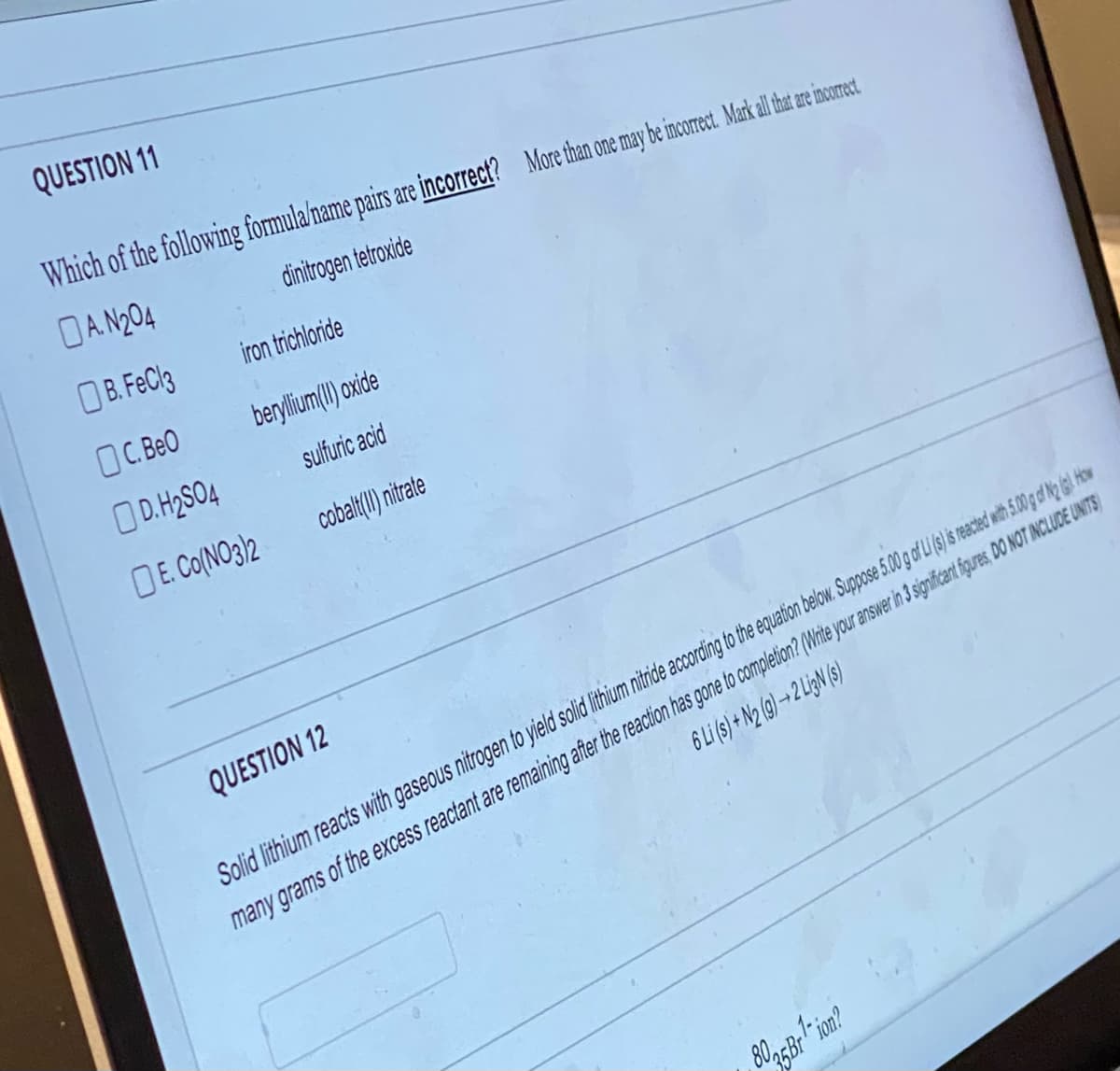
Transcribed Image Text:QUESTION 11
Which of the following formula/name pairs are incorrect? More than one may be incorrect. Markall hat are incorect.
A.N204
dinitrogen tetroxide
B. FeCl3
OC. BeO
iron trichloride
beryllium(II)oxide
sulfuric acid
cobalt(II) nitrate
D.H₂SO4
E. Co(NO3)2
QUESTION 12
6 Li (s) + N2 (9)→2 Li3N (s)
Solid lithium reacts with gaseous nitrogen to yield solid lithium nitride according to the equation below. Suppose 5.00 g of Li (s) is reacted with 5.00 g of N2 (g). How
many grams of the excess reactant are remaining after the reaction has gone to completion? (Write your answer in 3 significant figures, DO NOT INCLUDE UNITS)
8035 Br¹-ion?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
