Question 12 of 25 Submit What mass grams of nitric acid , HNO, is required to neutralize (completely react with) 4.30 |g of Ca(OH)2 according to the acid-base reaction: 2 HNO,(aq) + Ca(OH)2(aq) → 2 H,0(1) + Ca(NO3)2(aq) 0.0580 mol GatNO 4.30
Question 12 of 25 Submit What mass grams of nitric acid , HNO, is required to neutralize (completely react with) 4.30 |g of Ca(OH)2 according to the acid-base reaction: 2 HNO,(aq) + Ca(OH)2(aq) → 2 H,0(1) + Ca(NO3)2(aq) 0.0580 mol GatNO 4.30
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.77PAE
Related questions
Question
100%
im very confused :( can you please provide a step by step to help me thank you
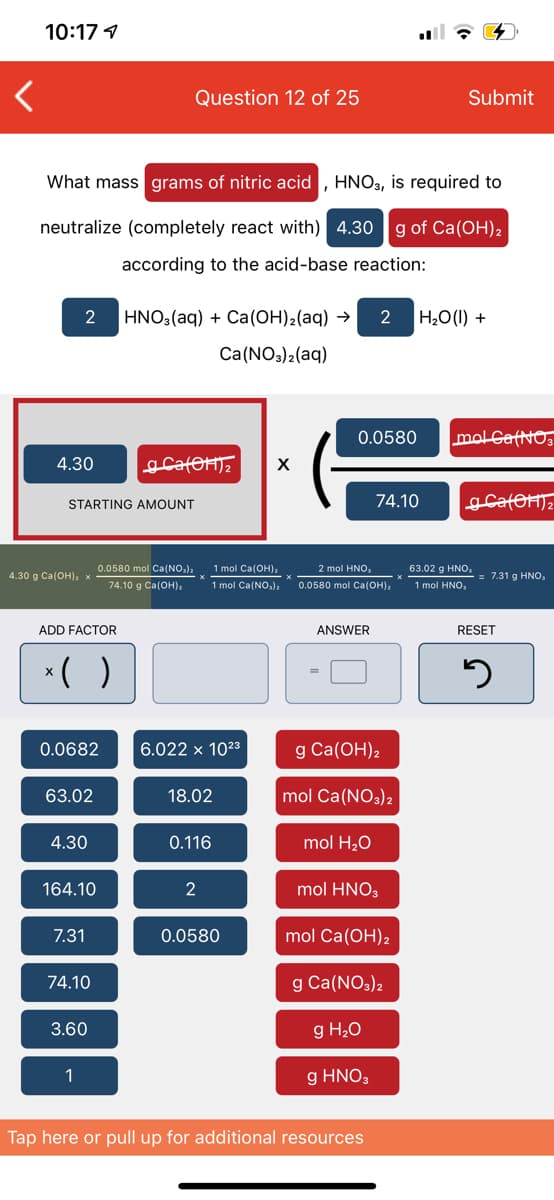
Transcribed Image Text:10:17 1
Question 12 of 25
Submit
What mass grams of nitric acid , HNO3, is required to
neutralize (completely react with) 4.30
g of Ca(OH)2
according to the acid-base reaction:
2
HNO:(aq) + Ca(OH)2(aq) →
2
H20(1) +
Ca(NO3)2(aq)
0.0580
molCafNO,
4.30
X
STARTING AMOUNT
74.10
0.0580 mol Ca(NO,),
1 mol Ca(OH),
2 mol HNO,
63.02 g HNO,
4.30 g Ca(OH), x
= 7.31 g HNO,
74.10 g Ca(OH):
1 mol Ca(NO,)a
0.0580 mol Ca(OH),
1 mol HNO,
ADD FACTOR
ANSWER
RESET
*( )
0.0682
6.022 x 1023
g Ca(ОН),
63.02
18.02
mol Ca(NO3)2
4.30
0.116
mol H20
164.10
2
mol HNO3
7.31
0.0580
mol Ca(OH)2
74.10
g Ca(NO3)2
3.60
g H20
1
g HNO3
Tap here or pull up for additional resources
-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning