Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter10: The Mole
Section10.4: Empirical And Molecular Formulas
Problem 61PP
Related questions
Question

Transcribed Image Text:QUESTION 14
How many moles of benzene C6H6 are present in 390 grams of benzene.
O 5 mol
4.3 mol
O 6.7 mol
O 8 mol
QUESTION 15
7.00 moles of N2 molecule contains how many N atoms?
O 8.44 X 1026 atom
O 4.00 X 1024 atom
O 8.44 X 1024 atom
O2.44 X 1024 atom
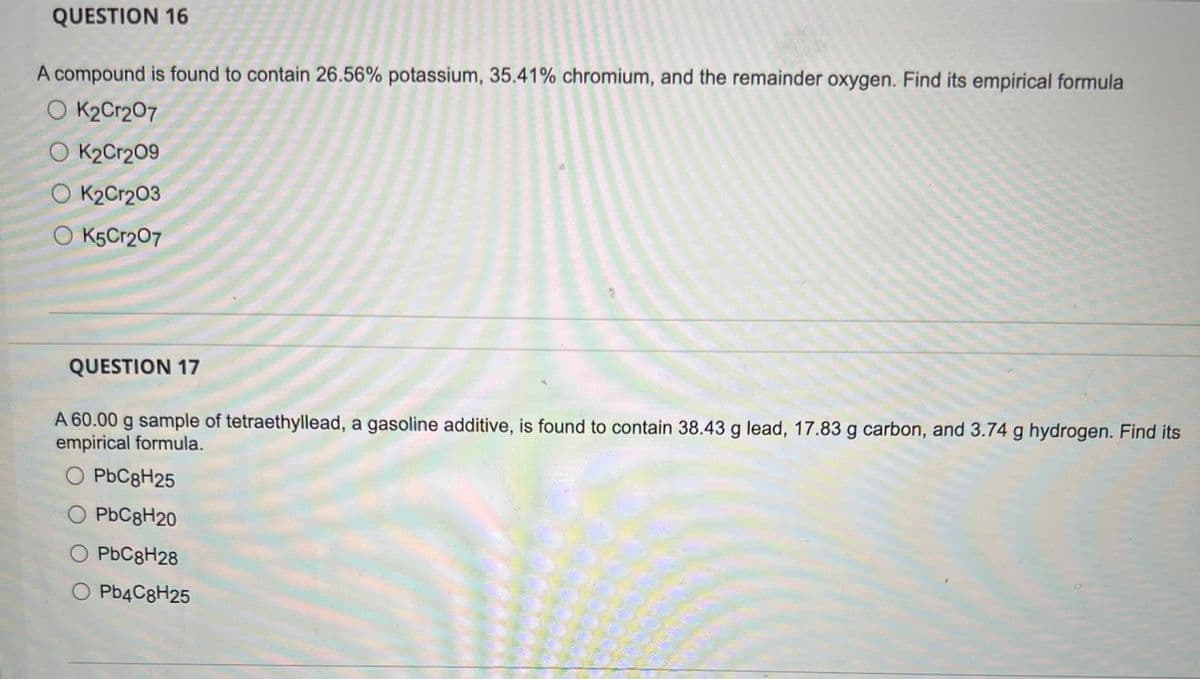
Transcribed Image Text:QUESTION 16
A compound is found to contain 26.56% potassium, 35.41% chromium, and the remainder oxygen. Find its empirical formula
O K2Cr2O7
O K₂Cr209
OK2Cr203
O K5Cr2O7
QUESTION 17
A 60.00 g sample of tetraethyllead, a gasoline additive, is found to contain 38.43 g lead, 17.83 g carbon, and 3.74 g hydrogen. Find its
empirical formula.
PbC8H25
O PbC8H20
O PbC8H28
O Pb4C8H25
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co