QUESTION 15 Match the following elementslions with the correct ground state electronic configurations: Jlithium A. 152 252 2p6 3s2 3p6 45² 3d10 4p5 Jargon В. 1s2 2s2 2p6 3s2 3p6 452 3d10 4p6 bromine C. 1s2 2s1 zinc D. Imercury 1s2 252 2p6 3s2 4p6 E. 1s2 252 2p6 3s2 3p6 4s² 3d10
QUESTION 15 Match the following elementslions with the correct ground state electronic configurations: Jlithium A. 152 252 2p6 3s2 3p6 45² 3d10 4p5 Jargon В. 1s2 2s2 2p6 3s2 3p6 452 3d10 4p6 bromine C. 1s2 2s1 zinc D. Imercury 1s2 252 2p6 3s2 4p6 E. 1s2 252 2p6 3s2 3p6 4s² 3d10
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 14ALQ
Related questions
Question
100%
15 # please
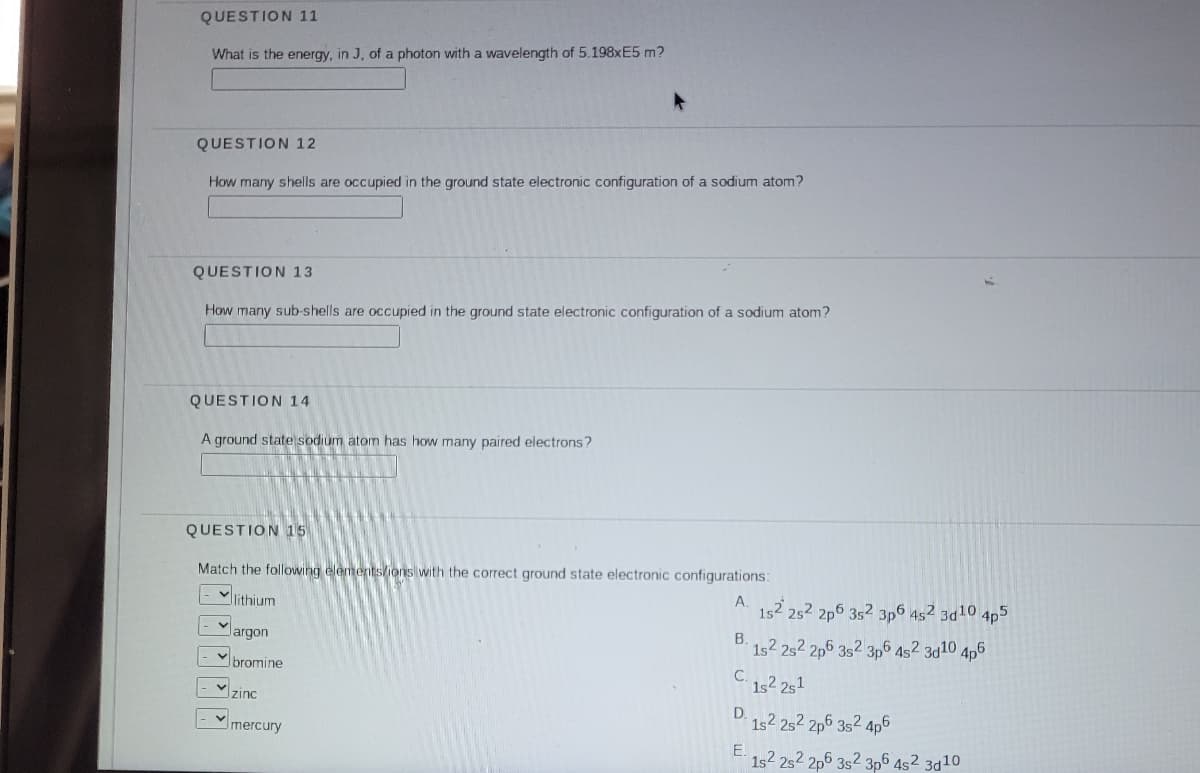
Transcribed Image Text:QUESTION 11
What is the energy, in J, of a photon with a wavelength of 5.198XE5 m?
QUESTION 12
How many shells are occupied in the ground state electronic configuration of a sodium atom?
QUESTION 13
How many sub-shells are occupied in the ground state electronic configuration of a sodium atom?
QUESTION 14
A ground state sodium atom has how many paired electrons?
QUESTION 15
Match the following elementslions with the correct ground state electronic configurations:
Vlithium
A.
152 252 2p6 352 3p6 452 3d10,
4p5
largon
1s2 2s2 2p6 3s2 3p6 452 3d10 4p6
bromine
C.
1s2 251
zinc
D.
1s2 252 2p6 352 4p6
Imercury
E.
1s2 2s2 2p6 3s2 3p6 4s² 3d10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning