The 2D particle in a box model can be used to describe the spectra of aromatic hydrocarbons. Assume a rectangular box that extends one bond length beyond the carbon atoms to contain the hydrogen atoms in the box. Hint: You will have to use some trigonometry basics to get the relevant dimensions. Calculate the first three electronic transitions for pyrene (bond length 1.42 Angstroms).
The 2D particle in a box model can be used to describe the spectra of aromatic hydrocarbons. Assume a rectangular box that extends one bond length beyond the carbon atoms to contain the hydrogen atoms in the box. Hint: You will have to use some trigonometry basics to get the relevant dimensions. Calculate the first three electronic transitions for pyrene (bond length 1.42 Angstroms).
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter15: Introduction To Electronic Spectroscopy And Structure
Section: Chapter Questions
Problem 15.58E
Related questions
Question
100%
V2
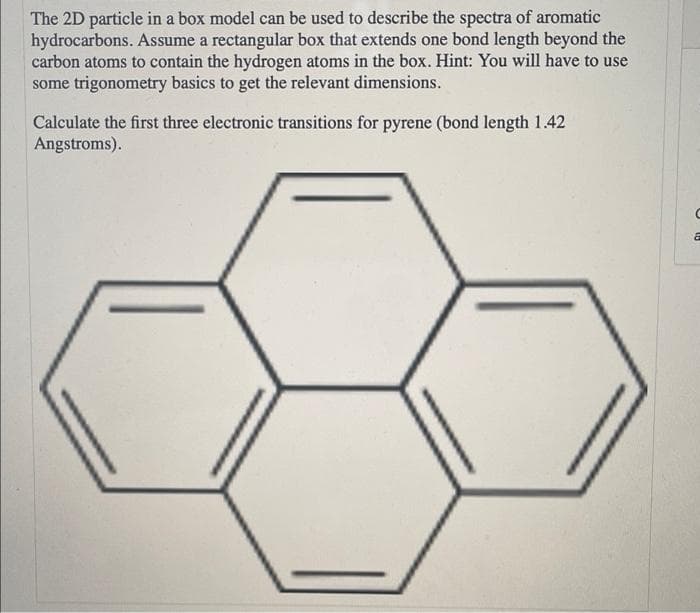
Transcribed Image Text:The 2D particle in a box model can be used to describe the spectra of aromatic
hydrocarbons. Assume a rectangular box that extends one bond length beyond the
carbon atoms to contain the hydrogen atoms in the box. Hint: You will have to use
some trigonometry basics to get the relevant dimensions.
Calculate the first three electronic transitions for pyrene (bond length 1.42
Angstroms).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning